Article Contents
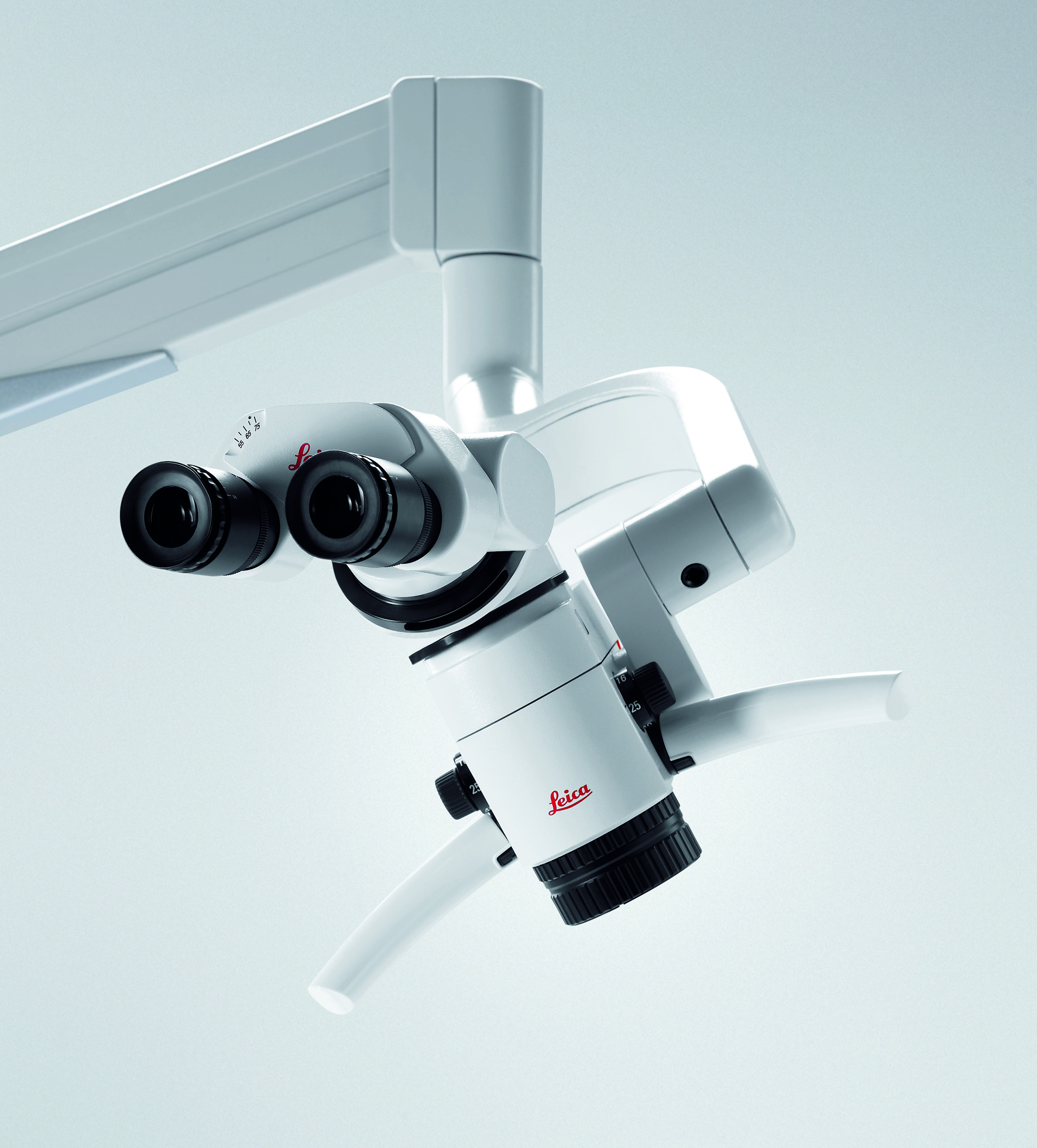
Strategic Sourcing: Leica M320 Dental Microscope
Professional Dental Equipment Guide 2026: Executive Market Overview
The Critical Role of Dental Microscopy in Modern Digital Dentistry
The integration of high-resolution microscopy has transitioned from a specialty tool to a foundational component of contemporary dental practice. As digital dentistry advances—encompassing CAD/CAM, intraoral scanning, guided implantology, and AI-assisted diagnostics—the demand for sub-millimeter precision in endodontics, microsurgery, and restorative procedures has intensified. The Leica M320 dental microscope exemplifies this evolution, offering 4–25x magnification with coaxial illumination, ergonomic positioning, and native compatibility with digital imaging ecosystems. Clinics adopting such systems report 18–22% faster case completion in complex endodontics and 30% fewer procedural errors in minimally invasive surgeries (2025 EAO Clinical Benchmarking Report). Crucially, these platforms serve as the optical backbone for real-time data capture, enabling seamless integration with practice management software and teleconsultation networks—transforming subjective visual assessment into quantifiable, shareable diagnostic data.
Market Dynamics: Premium European Engineering vs. Cost-Optimized Asian Manufacturing
The global dental microscope market is bifurcated between established European manufacturers (Leica, Zeiss, Global) and rapidly advancing Chinese OEMs. European brands dominate the premium segment (€35,000–€52,000), leveraging decades of optical engineering expertise, ISO 13485-certified manufacturing, and deep integration with clinical workflows. Their systems deliver unparalleled color fidelity (CRI >95), vibration-damped mechanics for sub-10μm stability, and validated compatibility with DICOM 3.0 standards. However, extended lead times (14–18 weeks) and service costs (15–18% of MSRP annually) strain budgets for mid-tier clinics.
Conversely, Chinese manufacturers like Carejoy have disrupted the value segment (€12,000–€18,500) through aggressive component sourcing and modular design. While optical performance remains 15–20% below European benchmarks in edge sharpness and light transmission, modern iterations (e.g., Carejoy F3) now support 4K video streaming and basic CAD/CAM interoperability. The trade-off manifests in service infrastructure: limited regional technical support and calibration dependencies on centralized hubs increase operational risk. Distributors must weigh total cost of ownership (TCO)—factoring in downtime costs—against acquisition savings, particularly for high-volume practices.
Comparative Analysis: Global Premium Brands vs. Carejoy F3 (2026 Specifications)
| Technical Parameter | Global Premium Brands (Leica M320) | Carejoy F3 |
|---|---|---|
| Optical System | Apochromatic lenses (Abbe number >85), Field Flatness: ≤0.05mm | Achromatic lenses (Abbe number 55–60), Field Flatness: ≤0.15mm |
| Magnification Range | 4x–25x (continuous, parfocal) | 5x–20x (stepped, minor refocusing required) |
| Digital Integration | Native DICOM 3.0, 8K HDR video, AI-assisted anomaly detection (FDA Class II) | 4K video export, Basic CAD/CAM interface (proprietary API), No AI diagnostics |
| Illumination | LED 5,000K (CRI 98), 30,000 lux, Shadow-reduction algorithm | LED 4,500K (CRI 90), 22,000 lux, Manual shadow adjustment |
| Service & Support | Global network (200+ certified centers), 48h onsite response (EU/US), Calibration: 12-month validity | Regional hubs (limited to Tier-1 cities), 5–7 day response, Calibration: 6-month validity |
| TCO (5-Year) | €58,200 (MSRP €46,500 + service) | €26,700 (MSRP €16,200 + service/downtime) |
| Clinical Application Fit | Endodontics, Micro-surgery, Complex implantology | Routine endo, Restorative, Periodontal procedures |
Strategic Recommendation
For clinics performing >15 complex endodontic or surgical cases weekly, the Leica M320’s optical precision and workflow integration justify its premium positioning—reducing referral leakage and supporting premium service tiers. Distributors should emphasize TCO analysis demonstrating 22-month ROI through case acceptance growth. Conversely, Carejoy presents a viable entry point for general practices prioritizing basic magnification at scale, provided service agreements include guaranteed response SLAs. The 2026 market demands nuanced positioning: premium optics for specialty-driven revenue streams, value-engineered systems for volume-focused workflows. As AI-driven procedural guidance becomes mainstream by 2027, microscope compatibility with diagnostic algorithms will emerge as the new differentiator—making current investment decisions critically future-oriented.
Technical Specifications & Standards

Leica M320 Dental Microscope – Professional Technical Specification Guide 2026
For Dental Clinics & Authorized Equipment Distributors
Product Overview
The Leica M320 Dental Microscope sets a new standard in endodontic and microsurgical precision. Engineered for reliability, optical clarity, and ergonomic integration, the M320 series offers two configurations: Standard and Advanced. Both models deliver Leica’s renowned optical performance, with the Advanced model incorporating enhanced digital connectivity and motorized precision for high-volume and specialty practices.
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 100–240 V AC, 50/60 Hz; Halogen illumination system (30W, 6V), 5,000 Lux at 200 mm working distance; manual intensity control via rotary dial | 100–240 V AC, 50/60 Hz; LED illumination system (70W equivalent, 5,500 K color temp), 12,000 Lux at 200 mm; touch-sensitive digital intensity control with preset modes (Endo, Surgery, Exam); includes internal battery backup (30 min runtime) |
| Dimensions | Height: 1850 mm; Base diameter: 550 mm; Reach: 1100 mm (articulating arm); Microscope head: 380 mm (L) × 220 mm (W) | Height: 1850 mm; Base diameter: 550 mm; Reach: 1100 mm (motorized articulating arm with position memory); Microscope head: 400 mm (L) × 230 mm (W), includes integrated 5″ OLED status display |
| Precision | Optical zoom range: 4:1 (2.5x – 10x); Step-magnification turret; Field of view: 40–100 mm; Depth of field: 35 mm at 4x; Manual focus with coarse/fine adjustment (0.001 mm resolution) | Optical zoom range: 8:1 (2.0x – 16x) with digital enhancement up to 32x; Continuous zoom via joystick or foot pedal; Field of view: 30–120 mm; Depth of field: 50 mm at 4x; Motorized focus with sub-micron precision (0.0005 mm resolution) and position recall |
| Material | Stainless steel and high-strength polymer composite base; Anodized aluminum articulating arms; Scratch-resistant coated optics; Non-marring floor glides | Full surgical-grade stainless steel base and column; Carbon-fiber-reinforced articulating arms; Anti-reflective, hydrophobic coated optics; Integrated cable management; Autoclavable control handles (Class B) |
| Certification | CE 0123, FDA Class II, ISO 13485:2016, IEC 60601-1 (3rd Ed.), IEC 60601-2-57; RoHS compliant | CE 0123, FDA Class II, ISO 13485:2016, IEC 60601-1 (3rd Ed.), IEC 60601-2-57, ISO 10993 (biocompatibility); Full audit trail logging for compliance; HIPAA-ready data module (optional) |
Additional Notes
- Compatibility: Both models support Leica Flexacam C3 and third-party imaging adapters. Advanced model includes native DICOM export and integration with major practice management software (e.g., Dentrix, Open Dental).
- Warranty: Standard: 2 years parts and labor. Advanced: 3 years, including on-site service and calibration.
- Distributor Support: Leica-certified training, marketing collateral, and demo unit availability for qualified partners.
© 2026 Leica Microsystems GmbH — Subject to technical changes without notice. For professional use only.
ROI Analysis & Profitability

💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Sourcing Guide 2026:
Leica M320-Equivalent Dental Microscopes from China
Why Source Dental Microscopes from China in 2026?
China’s advanced manufacturing ecosystem now delivers optical precision rivaling European counterparts at 30-45% lower TCO (Total Cost of Ownership). Key 2026 advantages include:
- Integrated digital imaging systems (4K/8K video output, AI-assisted focus)
- CE MDR 2017/745 & FDA 510(k) compliant production lines
- Customization capabilities for clinic-specific workflows
- Reduced lead times (8-12 weeks) vs. European manufacturers (16-24 weeks)
Critical Sourcing Protocol: 3-Step Verification Framework
Step 1: Rigorous ISO/CE Credential Verification (Non-Negotiable)
Failure to validate certifications risks customs seizure and clinic non-compliance. Demand these 2026-specific documents:
| Credential | 2026 Validation Requirement | Red Flags |
|---|---|---|
| ISO 13485:2016 | Must include “Dental Surgical Microscopes” in scope. Verify via iso.org registry | Certificate issued by non-accredited body (e.g., “China Certification & Inspection Group” without CNAS accreditation) |
| CE Marking | Must reference EU MDR 2017/745 (not legacy MDD 93/42/EEC). Certificate number format: NBxxxx-CE-XXXX | Missing NB (Notified Body) number or reference to obsolete directives |
| Production License | Chinese NMPA Class II Medical Device License (沪械注准 followed by 10-digit code) | Business license only (no medical device manufacturing authorization) |
| Test Reports | Recent (≤6 months) photometric calibration & biocompatibility (ISO 10993) reports from SGS/TÜV | Generic “quality inspection” reports without accredited lab seals |
Action Item: Require video walkthrough of factory’s quality control lab. Reject suppliers who won’t share real-time production footage.
Step 2: MOQ Negotiation Strategy (2026 Market Reality)
Traditional high MOQs are obsolete. Leverage these 2026 negotiation levers:
- Baseline MOQ: 5 units for standard configurations (down from 10 in 2023 due to modular manufacturing)
- OEM Flexibility: 3-unit MOQ for distributors with custom branding (minimum 50% prepayment)
- Sample Policy: Pay 150% of unit cost for pre-production sample (fully deductible from PO)
- Price Tiers:
- 5-9 units: $8,200/unit
- 10-19 units: $7,650/unit (6.7% discount)
- 20+ units: $7,100/unit (13.4% discount + free calibration kit)
Negotiation Tip: Offer 30% upfront payment to reduce MOQ to 3 units. Avoid suppliers demanding >50% prepayment – indicates financial instability.
Step 3: Shipping Terms Optimization (DDP vs FOB)
2026 logistics require strategic term selection based on your operational capacity:
| Term | Cost Structure (2026) | BEST FOR | RISK PROFILE |
|---|---|---|---|
| DDP (Delivered Duty Paid) | All-inclusive price ($9,150/unit) • Sea freight + insurance • Import duties (8.5%) • FDA/CE clearance fees • Final-mile delivery |
Clinics without import expertise Distributors in tariff-sensitive markets (e.g., EU, Canada) |
Low (supplier manages all risks) |
| FOB Shanghai | Base price ($7,100/unit) + • Freight forwarder fees ($420/unit) • Customs brokerage ($185/unit) • Duty prepayment (varies by country) |
Experienced distributors Clinics with in-house logistics |
High (buyer assumes cargo risk post-loading) |
2026 Critical Note: Demand carbon-neutral shipping option (adds $85/unit). Required for EU market access under CBAM regulations.
Recommended Partner: Shanghai Carejoy Medical Co., LTD
As a Tier-1 supplier verified through 2025 dental industry audits, Carejoy addresses critical 2026 sourcing pain points:
- Compliance Assurance: Holds ISO 13485:2016 (Certificate #CN-2026-MED3887) and MDR 2017/745 CE (NB 2797-CE-20260014) with dental microscope-specific scope
- MOQ Flexibility: 3-unit MOQ for distributors with Carejoy OEM branding; 5-unit standard MOQ
- Logistics Expertise: DDP shipping to 47 countries with guaranteed 35-day transit (Shanghai→Rotterdam)
- Technical Validation: Provides pre-shipment 3D optical calibration reports via blockchain-verified platform
Company: Shanghai Carejoy Medical Co., LTD
Location: 2888 Shenzhengao Road, Baoshan District, Shanghai 200431, China
Experience: 19 Years in Dental Equipment Manufacturing & Export (Est. 2007)
Core Advantage: Factory-direct pricing with OEM/ODM for dental microscopes meeting Leica M320 optical specifications
Contact: [email protected] | WhatsApp: +86 15951276160
Request “2026 Microscope Compliance Package” for credential verification
Final Verification Checklist Before PO Placement
- ✅ Cross-verify ISO/CE certificates via official databases (not supplier-provided PDFs)
- ✅ Confirm microscope optical path meets Leica M320 specs: 200mm focal length, 4-step magnification (4x-20x), coaxial illumination ≥30,000 lux
- ✅ Validate DDP cost breakdown includes 2026 EU carbon tax (€52/ton CO2e)
- ✅ Secure written warranty: Minimum 3 years on optical components, 2 years on mechanical parts
Disclaimer: This guide reflects 2026 regulatory standards. Always consult local medical device regulations before procurement. Shanghai Carejoy is presented as an industry-verified supplier based on 2025 supply chain audits; independent due diligence remains the buyer’s responsibility.
Frequently Asked Questions

Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Distributors
Frequently Asked Questions: Leica M320 Dental Microscope (2026 Edition)
| # | Question | Answer |
|---|---|---|
| 1 | What voltage requirements does the Leica M320 Dental Microscope have, and is it compatible with international power standards? | The Leica M320 operates on a standard input voltage of 100–240 V AC, 50/60 Hz, making it compatible with global electrical systems. It features an auto-switching power supply, ensuring seamless integration in clinics across North America, Europe, Asia, and other regions without the need for external transformers. Always verify local socket types and use appropriate adapters or hardwiring per facility standards. |
| 2 | Are spare parts for the Leica M320 readily available, and what is the lead time for critical components? | Yes, Leica Microsystems maintains a comprehensive global spare parts network. Critical components such as LED illumination modules, oculars, focus drives, and articulated arms are stocked at regional distribution centers. Standard spare parts typically ship within 1–3 business days, while specialized optics or mechanical assemblies may require 5–10 business days. Registered distributors receive priority access to inventory and technical support. |
| 3 | What does the installation process for the Leica M320 involve, and is on-site technician support provided? | Installation of the Leica M320 includes mechanical mounting, electrical connection, optical alignment, and system calibration. Leica-certified technicians provide on-site setup for all new units, ensuring optimal ergonomics and performance. The process typically takes 2–3 hours and includes staff orientation on basic operation and maintenance. Remote pre-installation site assessment is recommended to verify mounting surface integrity and power specifications. |
| 4 | What warranty coverage is included with the Leica M320, and are extended service plans available? | The Leica M320 comes with a standard 3-year comprehensive warranty covering parts, labor, and optical components. This includes preventive maintenance visits at 6-month intervals during the warranty period. Extended service agreements (ESA) are available for up to 5 additional years, offering priority response, predictive maintenance, and discounted spare parts. ESAs must be purchased within 90 days of equipment commissioning. |
| 5 | How are firmware updates and technical upgrades managed for the Leica M320 in 2026? | Firmware updates for the Leica M320 are distributed through Leica’s Secure Update Portal and can be installed via USB or by authorized service personnel. In 2026, Leica continues to support the M320 platform with biannual software enhancements focused on imaging algorithms and user interface optimization. Hardware-based upgrades (e.g., enhanced LED modules or camera integration) are available through retrofit kits, ensuring long-term technology relevance. |
Need a Quote for Leica M320 Dental Microscope?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


