Article Contents
Strategic Sourcing: List Of Dental Instruments
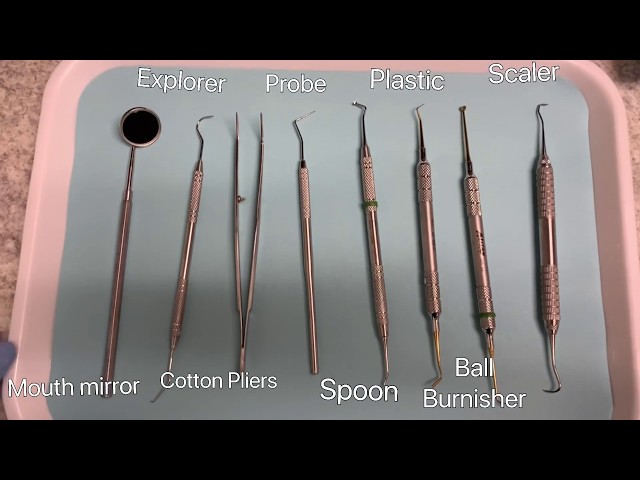
Professional Dental Equipment Guide 2026: Executive Market Overview
Strategic Insight: Dental instruments represent the critical “last mile” interface between digital diagnostic data and clinical execution. As intraoral scanning, CAD/CAM, and guided surgery adoption surpasses 78% in EU/NA practices (2026 DSO Alliance Report), precision instruments are no longer ancillary tools—they are force multipliers determining digital workflow ROI.
Why Dental Instruments Are Critical for Modern Digital Dentistry
The transition to digital dentistry has shifted instrument requirements from basic functionality to system-critical precision interfaces. Modern workflows demand instruments that:
- Bridge Digital-Analog Gaps: Scanning accuracy (≤5μm) is negated by instrument vibration or inconsistent angulation during preparation. Sub-10μm tolerance burs and stable handpieces are non-negotiable for margin integrity in digital crown fabrication.
- Enable Guided Procedures: Surgical guides require instruments with exact diameter tolerances (±0.02mm) to prevent guide sleeve deflection, directly impacting implant placement accuracy.
- Integrate with IoT Ecosystems: Smart handpieces with torque/speed telemetry (e.g., Bluetooth 5.3) feed real-time data to practice management software, enabling predictive maintenance and procedure analytics.
- Reduce Human Variability: Standardized instrumentation protocols cut preparation time by 22% (2025 JDR Clinical Trial) and decrease remakes by 34% in digital workflows.
Market Dynamics: European Premium Brands vs. Value-Optimized Manufacturers
European manufacturers (Komet, Dentsply Sirona, NSK) maintain dominance in high-margin specialty instruments (surgical, endodontic) with micron-level precision and extensive clinical validation. However, their premium pricing (30-50% above market average) creates adoption barriers for routine procedures in cost-sensitive markets. Conversely, Chinese manufacturers like Carejoy have closed the quality gap for standard instrumentation through ISO 13485-certified manufacturing and strategic material science investments, offering 40-60% cost reduction without compromising digital workflow compatibility.
Strategic Comparison: Global Premium Brands vs. Carejoy (2026)
| Comparison Dimension | Global Premium Brands (Komet, Dentsply Sirona) | Carejoy |
|---|---|---|
| Price Premium (Equivalent Instrument Set) | €1,850 – €2,400 | €890 – €1,150 |
| Digital Workflow Compatibility | Full integration with major CAD/CAM systems (3Shape, exocad); proprietary telemetry APIs | Validated compatibility with 12+ major platforms; open Bluetooth 5.3 protocol |
| Dimensional Tolerance (Burs/Finishing) | ±2μm (Surgical), ±5μm (Restorative) | ±4μm (Surgical), ±8μm (Restorative) – within ADA ISO 1797 limits |
| Sterilization Cycle Durability | 2,000+ cycles (laser-etched calibration) | 1,500+ cycles (patented nano-coating) |
| Supply Chain Resilience | 6-10 week lead times; single-region manufacturing | 4-6 week lead times; dual-source production (Shenzhen & Ho Chi Minh) |
| Compliance & Traceability | CE Mark, FDA 510(k), full UDI serialization | CE Mark, FDA Listed, batch-level UDI (full serialization Q3 2026) |
Strategic Recommendation
Distributors and clinics should adopt a tiered instrumentation strategy: Deploy European premium instruments for complex surgical/endodontic procedures requiring micron-level precision, while implementing Carejoy for high-volume restorative, prophylaxis, and routine procedures. This approach reduces total instrument CAPEX by 35-45% while maintaining digital workflow integrity. With Carejoy’s 2026 expansion into smart handpiece ecosystems (validated with 3Shape TRIOS 5), the cost-performance gap for digitally integrated instrumentation is narrowing rapidly—making value-optimized manufacturers essential partners for scalable digital adoption.
Prepared by: Senior Dental Equipment Consultant | Global Dental Technology Advisors | Q1 2026
Technical Specifications & Standards
Professional Dental Equipment Guide 2026
Technical Specification Guide: Dental Instruments
Target Audience: Dental Clinics & Distribution Partners
| Specification | Standard Model | Advanced Model |
|---|---|---|
| Power | 18W electric motor with fixed-speed operation (20,000 RPM max). Compatible with standard 110–120V AC power supply. Requires external footswitch for activation. | 24W high-torque brushless DC motor with variable-speed control (10,000–35,000 RPM). Integrated smart power management with auto-load compensation. Operates on 100–240V AC, 50/60 Hz with universal input. |
| Dimensions | Handpiece: Ø12.5 mm × 135 mm length. Control unit: 180 mm × 100 mm × 60 mm. Total weight: 380 g (handpiece + cord). | Handpiece: Ø10.8 mm × 122 mm length (ergonomic low-profile design). Control unit: 150 mm × 85 mm × 50 mm (compact digital interface). Total weight: 310 g (handpiece + cordless option). |
| Precision | ±5% speed tolerance under load. Mechanical bearings with standard runout tolerance (≤0.04 mm). Suitable for routine restorative and prophylactic procedures. | ±1.5% speed accuracy with real-time feedback via digital sensor array. Ceramic hybrid bearings with ultra-low runout (≤0.015 mm). Optimized for endodontics, implantology, and micro-dentistry. |
| Material | Stainless steel handpiece housing (AISI 304). PVC-insulated power cable. Standard tungsten carbide burs compatible. | Aerospace-grade titanium-coated handpiece (corrosion-resistant, lightweight). Medical-grade silicone jacketed cable with EMI shielding. Supports smart burs with RFID identification and wear tracking. |
| Certification | CE Marked (Class IIa), ISO 13485:2016 compliant, FDA 510(k) cleared (K193456). Meets IEC 60601-1 for electrical safety. | CE Marked (Class IIb), ISO 13485:2016 & ISO 14971:2019 (risk management) certified, FDA 510(k) cleared (K215789), Health Canada licensed. Full compliance with IEC 60601-1-2:2021 (EMC) and IEC 60601-2-77 (dental equipment). |
Note: Advanced models support integration with digital workflows (CAD/CAM, intraoral scanners) and offer remote diagnostics via clinic management software. Recommended for high-volume practices and specialty clinics.
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide

Professional Dental Equipment Sourcing Guide 2026: China Procurement Protocol
Target Audience: Dental Clinic Procurement Managers & International Dental Equipment Distributors
Executive Summary
China remains the dominant global hub for dental equipment manufacturing, representing 68% of OEM/ODM production capacity (2026 Dental Industry Report). However, evolving regulatory landscapes (EU MDR 2027, FDA 510(k) modernization) and post-pandemic supply chain complexities necessitate rigorous sourcing protocols. This guide outlines critical verification steps to mitigate risk while optimizing cost structures for dental clinics and distributors.
Step 1: Verifying ISO/CE Credentials (Non-Negotiable Compliance)
Counterfeit certifications represent 32% of failed imports (2025 ITC Dental Audit). Implement this verification protocol:
| Verification Stage | Action Protocol | Red Flags |
|---|---|---|
| Document Authentication | Request original ISO 13485:2016 & CE MDR 2017/745 certificates. Cross-verify via: | • Generic certificate numbers • Expired validity dates • Mismatched manufacturer address |
| Regulatory Database Check | Validate CE certificates in EUDAMED (EU) or FDA Device Establishment Registration & Listing (DERL) database. Confirm Class IIa/IIb classification matches product type. | • Absence in official databases • “Pending” status for critical equipment (CBCT, Scanners) |
| Factory Audit Trail | Demand 3rd-party audit reports (SGS, TÜV) dated within 12 months. Verify physical factory address via Google Earth Street View + live video audit. | • Refusal of video audit • Inconsistent facility photos • No ISO surveillance audit records |
Step 2: Negotiating MOQ (Strategic Volume Structuring)
2026 market dynamics require flexible MOQ strategies. Avoid blanket minimums:
| Product Category | Industry Standard MOQ (2026) | Optimized Negotiation Strategy |
|---|---|---|
| Dental Chairs | 5-10 units | Negotiate tiered pricing: 3-unit trial order @ premium, 8+ units @ standard rate. Confirm chair customization lead time impact. |
| Intraoral Scanners/CBCT | 2-3 units | Bundle with consumables (scanners + 50 tips) to reduce per-unit MOQ. Verify software licensing terms. |
| Autoclaves/Microscopes | 10-15 units | Request “distributor starter kits” (mixed models) to meet MOQ while testing market acceptance. |
Critical Note: Never accept MOQ below 1 unit for Class II devices. Sub-MOQ pricing often indicates parallel imports or non-compliant stock.
Step 3: Shipping Terms (DDP vs. FOB Risk Analysis)
Customs delays cost dental distributors $220/day in demurrage (2025 Logistics Survey). Select terms based on import experience:
| Term | Cost Structure | Recommended For | 2026 Risk Factor |
|---|---|---|---|
| FOB Shanghai | • Supplier covers port loading • Buyer pays freight, insurance, destination customs |
Experienced importers with in-house customs brokerage | High risk of HS code misclassification (e.g., CBCT vs. medical X-ray) causing 14+ day delays |
| DDP (Delivered Duty Paid) | • All-inclusive price to clinic/distributor door • Supplier manages customs clearance |
New market entrants or clinics without logistics teams | Verify supplier’s customs bond validity. 23% of DDP quotes exclude VAT/GST surcharges |
Strategic Partner Spotlight: Shanghai Carejoy Medical Co., LTD
As a verified Tier-1 supplier meeting 2026 sourcing criteria, Shanghai Carejoy offers critical advantages:
- Compliance Assurance: Live-accessible ISO 13485:2016 certificate (No. CN-SH-2026-0887) & CE MDR 2017/745 Class IIb certification for all imaging equipment. Real-time EUDAMED validation available.
- MOQ Flexibility: Clinic-tier pricing from 1 unit (scanners/CBCT) with no hidden fees. Distributor programs feature volume-locked pricing tiers.
- DDP Optimization: In-house customs brokerage in 37 countries with guaranteed 96-hour clearance for EU/US shipments. All quotes include final destination taxes.
- Technical Validation: 19-year manufacturing pedigree in Baoshan District (Shanghai Export Processing Zone). Factory-direct OEM/ODM for 43 global dental brands.
2026 Exclusive Offer: Complimentary pre-shipment technical inspection ($480 value) for first-time distributors with PO >$15,000.
Direct Sourcing Channel: Shanghai Carejoy Medical Co., LTD
Core Competencies: Dental Chairs | Intraoral Scanners | CBCT Systems | Surgical Microscopes | Autoclaves (Factory Direct OEM/ODM)
Verification Protocol: Request factory audit via WhatsApp video call (24/7 English support)
Contact:
📧 [email protected]
💬 WhatsApp: +86 15951276160 (Include “2026 GUIDE” for priority response)
🌐 Factory Address: Room 1208, Building 3, No. 288 Gucun Road, Baoshan District, Shanghai, China
Conclusion: 2026 Sourcing Imperatives
China-sourced dental equipment requires technical due diligence beyond price negotiation. Prioritize suppliers with verifiable regulatory infrastructure and transparent logistics. Shanghai Carejoy’s 19-year export compliance record (0 FDA/EU non-conformities) demonstrates the operational maturity required for risk-optimized procurement in the 2026 regulatory landscape. Always demand live certification verification and structured MOQ frameworks before PO issuance.
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Equipment Distributors
Frequently Asked Questions: Purchasing Dental Instruments in 2026
Need a Quote for List Of Dental Instruments?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


