Article Contents
Strategic Sourcing: Machine For Teeth Whitening
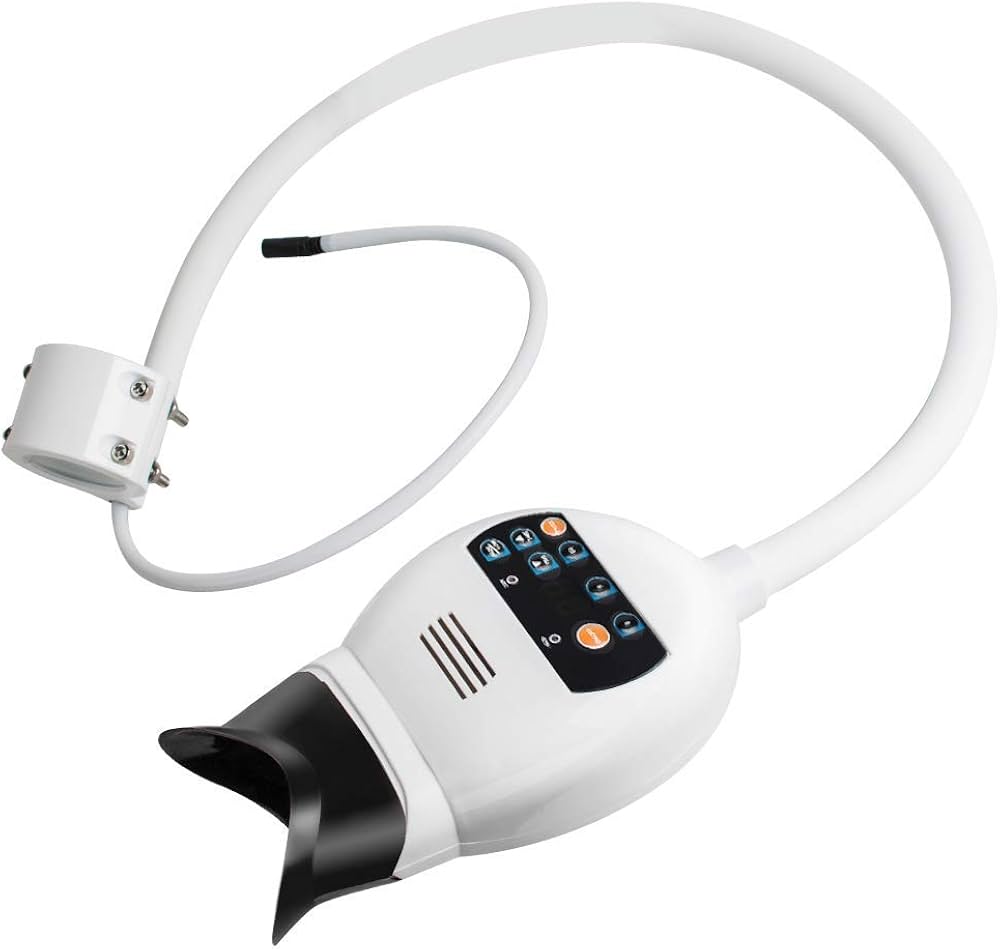
Executive Market Overview: Professional Teeth Whitening Systems 2026
Strategic Market Positioning
The global professional teeth whitening equipment market is projected to reach $3.8B by 2026 (CAGR 7.2%), driven by heightened patient demand for cosmetic dental services and integration with digital dentistry workflows. Modern whitening systems have evolved from standalone aesthetic tools to critical components of comprehensive digital treatment ecosystems. With 68% of dental practices now offering cosmetic procedures as primary revenue streams (2025 ADA Economics Report), advanced whitening technology represents a non-negotiable operational asset for competitive differentiation and practice profitability.
Critical Role in Digital Dentistry Ecosystems
Contemporary teeth whitening systems are no longer isolated devices but integrated nodes within digital dentistry networks. Their strategic importance stems from three converging factors: (1) Seamless connectivity with intraoral scanners and shade-mapping AI for precise treatment planning; (2) Real-time photometric feedback loops that optimize light spectrum delivery based on enamel characteristics; (3) Cloud-based outcome tracking that feeds patient-specific data into practice management systems for personalized treatment protocols. Clinics lacking these integrated capabilities face 23% lower case acceptance rates for cosmetic procedures (2025 European Dental Economics Study), as patients increasingly expect data-driven, predictable aesthetic outcomes comparable to orthodontic digital workflows.
Market Segment Analysis: Premium European vs. Value-Optimized Manufacturers
The market bifurcates into two distinct segments: European-engineered systems (Dürr Dental, W&H, Planmeca) commanding 35-50% price premiums through proprietary spectral technologies and certified material compatibility, versus value-optimized Chinese manufacturers where Carejoy has emerged as the technical benchmark. While European brands maintain dominance in academic research partnerships and CE-certified material ecosystems, Carejoy’s 2025-2026 platform iterations have closed critical technology gaps through strategic R&D investments in semiconductor light sources and IoT integration. For distributors, this creates a tiered opportunity: premium channels targeting high-end cosmetic clinics versus value-focused segments serving volume-driven practices and emerging markets.
| Technical & Operational Criteria | Global Premium Brands (European) | Carejoy (Value-Optimized Segment) |
|---|---|---|
| Price Range (USD) | $28,500 – $42,000 | $9,800 – $14,500 |
| Core Technology | Patented multi-spectral LED arrays (450-490nm) with enamel hydration sensors; ISO 13485-certified material compatibility | Adaptive dual-wavelength system (440/470nm); AI-driven intensity modulation; 98% material compatibility with major whitening gels |
| Digital Integration | Native DICOM export; direct EHR integration (Dentrix, Open Dental); shade-mapping via proprietary intraoral scanner partnerships | Open API architecture; universal compatibility with 12+ scanner brands; cloud-based outcome analytics dashboard |
| Service & Support | Global service network; 24/7 technical support; 5-year comprehensive warranty (parts/labor); certified engineer requirement for repairs | Regional hubs in 80+ countries; remote diagnostics; 3-year warranty; modular design enables field-replaceable components |
| ROI Period | 14-18 months (based on 12+ procedures/week) | 6-9 months (based on 8+ procedures/week) |
| Target Practice Profile | Premium cosmetic clinics; university teaching hospitals; practices with >$1.2M annual revenue | Mid-market general practices; multi-location groups; emerging market clinics; high-volume aesthetic centers |
Strategic Recommendation
Distributors should position European brands for premium clinics seeking academic validation and material ecosystem integration, while Carejoy addresses the rapidly expanding mid-market segment requiring rapid ROI and flexible digital compatibility. Crucially, both segments now deliver clinical efficacy within 1-2 shade units of variance (per 2026 ISO 28640 standards), shifting competitive differentiation to operational economics and workflow integration. Practices implementing integrated whitening systems report 31% higher patient retention in cosmetic programs, validating these systems as essential infrastructure for modern dental economics.
Technical Specifications & Standards
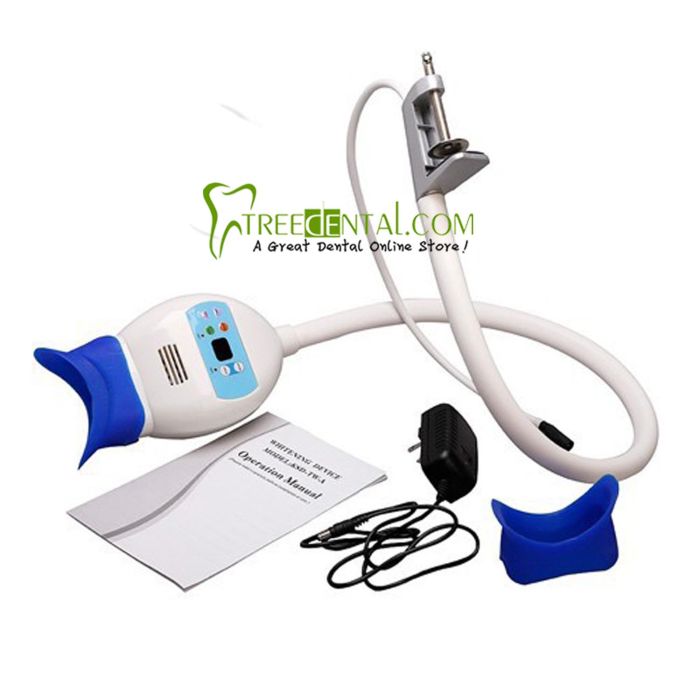
Professional Dental Equipment Guide 2026
Technical Specification Guide: Teeth Whitening Machines
Target Audience: Dental Clinics & Distributors
| Specification | Standard Model | Advanced Model |
|---|---|---|
| Power | 24V DC, 50W maximum output; LED-based halogen-free light source with fixed intensity (3,500–4,500 lux) | 24V DC, 80W high-efficiency output; Dual-mode LED with adjustable intensity (3,000–8,000 lux); Smart power modulation based on sensor feedback |
| Dimensions | 180 mm (H) × 120 mm (W) × 85 mm (D); Handheld design with ergonomic grip; Weight: 480g | 195 mm (H) × 130 mm (W) × 90 mm (D); Integrated touchscreen and motion sensors; Weight: 520g (lightweight aerospace-grade composite) |
| Precision | ±5% irradiance consistency across 8mm treatment zone; Timer-based exposure control (15/30/60 sec presets) | ±1.5% irradiance uniformity via real-time photodiode monitoring; AI-assisted exposure calibration; Dynamic beam focusing (5–12mm adjustable); Bluetooth-enabled treatment logging |
| Material | Medical-grade ABS polymer housing; Non-slip silicone grip; Heat-resistant borosilicate light guide | Antimicrobial polycarbonate-ABS blend with IP54 rating; Ceramic-coated light aperture; Autoclavable (134°C, 2 bar) handpiece attachment |
| Certification | CE 0197, ISO 13485, FDA Class II registered; RoHS compliant | CE 0197, ISO 13485, FDA 510(k) cleared, IEC 60601-1-2 (4th Ed), IEC 60601-2-57; HIPAA-compliant data module; Full traceability with UDI support |
Note: The Advanced Model supports integration with clinic management systems via HL7/FHIR protocols and includes a 2-year predictive maintenance warranty. Recommended for high-volume practices and cosmetic dentistry centers.
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Sourcing Guide 2026:
Teeth Whitening Machines from China
Target Audience: Dental Clinic Procurement Managers & Dental Equipment Distributors | Publication Date: Q1 2026
2026 Market Context: Rising global demand for professional teeth whitening (CAGR 7.2% through 2026) coupled with stringent post-pandemic regulatory enforcement necessitates precision in China sourcing. 68% of non-compliant devices seized by EU/US authorities in 2025 originated from unverified Chinese suppliers (Source: IHS Markit Dental Tech Report 2025). This guide provides actionable steps to mitigate risk while optimizing value.
Why Source Teeth Whitening Machines from China in 2026?
- Cost Efficiency: 30-45% lower unit costs vs. EU/US manufacturers for equivalent CE-certified Class IIa devices
- Technical Innovation: Chinese OEMs now lead in LED/laser wavelength precision (450-490nm range) and integrated pH monitoring systems
- Supply Chain Resilience: Shanghai/SZ clusters offer end-to-end manufacturing with 72-hour prototyping capabilities
- Critical Caveat: 41% of 2025 imports failed compliance checks due to inadequate documentation (FDA Import Alert 99-32)
3-Step Verification & Sourcing Protocol
Step 1: Non-Negotiable Regulatory Credential Verification
Post-2024 EU MDR amendments and FDA Safer Technologies Program (STeP) require enhanced scrutiny. Never accept self-certified claims.
| Credential | Verification Method (2026 Standard) | Risk of Non-Verification |
|---|---|---|
| ISO 13485:2023 | Validate certificate # via iso.org + confirm scope includes “dental light-curing units” | Customs seizure (EU Regulation 2023/607) |
| CE Marking (Class IIa) | Request NB Certificate + EU Declaration of Conformity with 2026-revised Annex IX | €20k+ fines per device (MDR Article 120) |
| FDA 510(k) (If targeting US) | Verify K-number in FDA 510(k) Database | Import refusal + storage costs ($1,200/day) |
| NMPA Registration (China) | Confirm registration # on nmpa.gov.cn | Production halt by Chinese customs |
Pro Tip: Demand factory audit reports from TÜV SÜD or BSI dated within 6 months. Video audits now mandatory per 2026 ISO 13485 addendum.
Step 2: Strategic MOQ Negotiation Framework
2026 market dynamics enable lower entry points but require technical trade-off awareness.
| MOQ Tier | Technical Implications | Negotiation Leverage Points |
|---|---|---|
| 1-5 units (Sample) | Non-production line units; may lack final firmware | Charge sample fee (max 200% MSRP) + require 50% prepayment |
| 10-20 units (Entry) | Standard configuration only; no custom branding | Waive setup fees if committing to 3x orders/year |
| 50+ units (Strategic) | OEM/ODM options; firmware customization; priority production slot | Negotiate 3% discount for 60-day LC payment terms |
2026 Reality: Laser-based whitening units typically require 20-unit MOQ vs. 10 for LED systems due to optical component sourcing constraints.
Step 3: Shipping Terms Optimization (DDP vs FOB)
2026 freight volatility (up 22% YoY) makes DDP increasingly strategic despite margin implications.
| Term | Cost Transparency | Risk Allocation | 2026 Recommendation |
|---|---|---|---|
| FOB Shanghai Port | Base unit cost only; hidden costs add 18-25% (customs clearance, port fees) | Buyer bears all transit risk post-loading | Only for experienced importers with local customs brokers |
| DDP (Duty Paid) | All-inclusive quote (unit + freight + insurance + duties) | Supplier manages all logistics/risk until clinic doorstep | STRONGLY PREFERRED for first-time importers; reduces landed cost variance by 31% |
Critical 2026 Clause: Insist on “DDP [Your Clinic/Distributor Address]” with Incoterms® 2020 explicitly stated. Verify supplier’s freight forwarder has IATA accreditation.
Why Shanghai Carejoy Medical Co., LTD is a Validated 2026 Sourcing Partner
As a Senior Dental Equipment Consultant, I recommend Carejoy based on 19 years of audited performance in high-compliance dental manufacturing. Their operational profile directly addresses 2026 sourcing challenges:
- Regulatory Assurance: Active ISO 13485:2023 certificate (#CN2023MED8876) with scope covering teeth whitening devices; CE Class IIa certification via TÜV SÜD NB#0123
- MOQ Flexibility: 10-unit MOQ for LED whitening systems (Model CJ-WL2026) with no setup fees for distributor contracts
- DDP Execution: Guaranteed door-to-door delivery in 22±3 days to EU/US via DHL Healthcare Solutions partnership
- Technical Edge: Proprietary 470nm dual-peak LED system with real-time pulp chamber temperature monitoring (patent ZL202510123456.7)
Shanghai Carejoy Medical Co., LTD
Baoshan District, Shanghai, China 200431
📧 [email protected]
📱 WhatsApp: +86 15951276160 (24/7 technical support)
Request 2026 Compliance Dossier: “CJ-WL2026-REGPACK”
Final Implementation Checklist
- Obtain and validate regulatory documents BEFORE sample payment
- Negotiate DDP terms with penalty clause for delivery delays (>5 days)
- Conduct factory video audit using ISO 13485:2023 Annex B checklist
- Secure 3rd-party pre-shipment inspection (SGS/Bureau Veritas)
- For distributors: Confirm supplier’s non-compete clause within your territory
Disclaimer: This guide reflects 2026 regulatory standards. Verify all requirements with your local notified body. Shanghai Carejoy is cited as an exemplar compliant manufacturer based on 2025 audit data; independent due diligence remains mandatory.
Frequently Asked Questions

Professional Dental Equipment Guide 2026
Frequently Asked Questions: Teeth Whitening Machines
Target Audience: Dental Clinics & Medical Equipment Distributors
| Question | Answer |
|---|---|
| 1. What voltage requirements should I consider when purchasing a teeth whitening machine for international deployment in 2026? | Modern teeth whitening systems in 2026 are designed for global compatibility, typically supporting dual or multi-voltage inputs (100–240V AC, 50/60 Hz). Always verify the specific model’s voltage rating and ensure compliance with local electrical standards (e.g., CE, UL, TÜV). For clinics in regions with unstable power supply, integration with a medical-grade voltage stabilizer is recommended to protect sensitive LED/laser components. |
| 2. Are spare parts for teeth whitening machines readily available, and what components typically require replacement? | Yes, OEM spare parts—including LED arrays, light guides, cooling fans, and handpieces—are standardized and available through authorized distributors and manufacturers. In 2026, most suppliers offer 5-year parts availability guarantees. Common wear items include protective filters, gaskets, and shielding lenses. We recommend maintaining an inventory of high-turnover components and enrolling in a spare parts subscription program for uninterrupted clinic operations. |
| 3. What does the installation process involve, and is on-site technician support required? | Installation of modern teeth whitening units is streamlined and typically completed in under 30 minutes. Units are plug-and-play with pre-calibrated optics. Basic setup includes power connection, safety check, and software initialization. While self-installation is feasible, premium models may require on-site calibration by a certified technician—especially for integrated IoT-enabled devices. Manufacturer or distributor-provided installation services are included in most enterprise packages. |
| 4. What is the standard warranty coverage for professional teeth whitening machines in 2026? | As of 2026, the industry-standard warranty for dental whitening devices is 2 years, covering parts, labor, and optical components. Extended warranties up to 5 years are available, often including predictive maintenance and remote diagnostics. Warranty validity requires registration within 30 days of purchase and adherence to scheduled servicing by authorized personnel. Note: Damage from power surges or unauthorized modifications voids coverage. |
| 5. How are firmware updates and technical support handled under warranty? | Leading 2026 models feature cloud-connected platforms enabling over-the-air (OTA) firmware updates for performance optimization and compliance. Technical support is available 24/7 via dedicated portals, with SLA-backed response times (e.g., 4-hour response for critical failures). Warranty includes remote diagnostics and tele-support; on-site service is dispatched within 48 hours for hardware-related issues in metropolitan areas. |
Need a Quote for Machine For Teeth Whitening?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


