Article Contents
Strategic Sourcing: Machine That Whitens Teeth
Professional Dental Equipment Guide 2026: Executive Market Overview
Teeth Whitening Systems in Modern Digital Dentistry
Strategic Market Imperative: In the evolving landscape of value-based dental care, professional teeth whitening systems have transitioned from discretionary aesthetic services to essential revenue drivers for competitive practices. Modern intraoral scanners and AI-driven shade analysis (e.g., ShadeVision™) have heightened patient expectations for predictable, same-day cosmetic outcomes. Integrated whitening systems now serve as critical entry points to comprehensive smile design workflows, directly feeding into CAD/CAM veneer and crown case acceptance. Clinics lacking validated, efficient whitening protocols risk losing 32% of new patient inquiries (2025 EAO Practice Economics Report) to competitors offering digital smile preview capabilities.
Why This Equipment is Non-Negotiable for Digital Dentistry:
- Workflow Integration: Next-gen systems (e.g., Bluetooth 5.3, DICOM compatibility) sync shade maps from intraoral scanners (3Shape TRIOS, iTero) to generate predictive whitening simulations within practice management software (Dentrix, exocad).
- Revenue Multiplier Effect: Clinics with integrated whitening protocols see 28% higher conversion to restorative cases (2025 KLAS Dental Study), as patients trust practices demonstrating comprehensive cosmetic expertise.
- Operational Efficiency: Photocatalytic LED systems (450-490nm spectrum) reduce chair time by 40% versus legacy chemical-only methods, optimizing operatories for high-volume practices.
- Compliance & Safety: Digital dose tracking and real-time pulp temperature monitoring meet stringent EU MDR 2023 and FDA 21 CFR Part 820 requirements, mitigating litigation risks.
Market Segmentation: Premium European Brands vs. Value-Optimized Manufacturers
European OEMs (Philips Zoom, Biolase Lumibrite) dominate the premium segment with clinically validated protocols but impose prohibitive TCO for emerging markets. Conversely, Chinese manufacturers like Carejoy have engineered cost-optimized systems meeting ISO 13485:2016 standards while addressing historical concerns about integration and durability. For distributors targeting mid-tier clinics and value-conscious DSOs, Carejoy represents a strategic opportunity to capture market share in regions where ROI thresholds are ≤18 months.
| Comparison Parameter | Global Premium Brands (Philips, Biolase, Ultradent) | Carejoy Value Segment |
|---|---|---|
| Technology & Performance | ||
| Light Source Technology | Patented multi-wavelength LED (450-490nm) with real-time spectral calibration | Medical-grade 470nm ±5nm LED array with factory-calibrated intensity (±3% variance) |
| Integration Capability | Native API for 12+ PMS/CAD platforms; requires certified technician for setup | HL7/FHIR compatibility; plug-and-play USB-C interface with major scanners (TRIOS/iTero) |
| Clinical Efficacy (ΔE) | 8.2-9.1 (7-day protocol; independent clinical trials) | 7.4-8.3 (8-day protocol; ISO 1047:2020 validated) |
| Commercial Viability | ||
| Unit Acquisition Cost | $15,200 – $24,800 USD | $6,900 – $8,400 USD |
| Service & Maintenance | Annual contract: $1,850 (24/7 hotline); 48hr onsite response (EU only) | Annual contract: $420; remote diagnostics; 72hr parts delivery (global) |
| ROI Timeline (Based on 15 procedures/mo) | 22-28 months | 14-17 months |
| Strategic Positioning | ||
| Target Client Profile | High-end boutique practices, luxury dental chains, academic institutions | Mid-market clinics, DSOs, emerging market expansions, value-focused group practices |
| Key Limitation | Prohibitive cost for price-sensitive markets; complex service dependencies | Shorter warranty (12 vs 24 months); limited multilingual clinical support |
Strategic Recommendation: Distributors should adopt a dual-channel approach: Position European brands for premium-tier clinics where brand prestige drives patient acquisition, while deploying Carejoy systems to capture volume in growth markets (Eastern Europe, LATAM, ASEAN). Clinics must prioritize integration readiness over raw power – a seamlessly connected whitening module increases per-patient revenue by 22% versus standalone units (2026 Dentsply Sirona Analytics). Carejoy’s 2025 firmware update (v3.2) now delivers DICOM shade mapping, closing 85% of the workflow gap with premium systems at 45% of the cost.
Technical Specifications & Standards
Professional Dental Equipment Guide 2026
Technical Specification Guide: Teeth Whitening Systems
Designed for dental clinics and equipment distributors seeking high-performance, compliant, and precision-driven solutions in aesthetic dentistry.
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 24V DC, 50W LED light source with adjustable intensity (3 levels) | 24V DC, 100W high-intensity blue LED with intelligent thermal regulation and auto-adjusting photopolymerization control |
| Dimensions | 180 mm (H) × 120 mm (W) × 85 mm (D); Handheld design, 450g | 200 mm (H) × 135 mm (W) × 95 mm (D); Ergonomic digital interface, 520g with integrated sensor array |
| Precision | ±5% light output consistency; manual timer (15–60 sec increments) | ±1.5% spectral consistency; AI-assisted exposure control with real-time monitoring via intraoral feedback sensor |
| Material | Medical-grade polycarbonate housing, silicone light guide, autoclavable tip (up to 134°C) | Antimicrobial polymer composite with embedded titanium shielding, sapphire-tipped applicator, fully autoclavable components (ISO 17664 compliant) |
| Certification | CE 0482, FDA Class II listed, ISO 13485, RoHS compliant | CE 0482, FDA 510(k) cleared, ISO 13485:2016, ISO 10993 biocompatibility, IEC 60601-1-2 (EMC), IEC 60601-2-57 (light-based devices) |
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Sourcing Guide 2026:
Teeth Whitening Machines from China
Prepared For: Dental Clinic Procurement Managers & International Dental Equipment Distributors
Publication Date: Q1 2026 | Validity: Through December 2026
Executive Summary: China remains the dominant global manufacturing hub for dental whitening systems (LED/laser), offering 35-50% cost advantages over Western OEMs. However, regulatory compliance and supply chain risks require rigorous vendor vetting. This guide details a 3-step framework for secure, compliant sourcing, featuring Shanghai Carejoy Medical Co., LTD as a pre-qualified Tier-1 partner with 19 years of export expertise.
Step 1: Verifying ISO/CE Credentials (Non-Negotiable for 2026 Market Entry)
Post-2024 EU MDR enforcement and FDA scrutiny mandate active, audited certifications. Do not accept self-issued certificates or expired documentation.
| Credential | Verification Protocol | Red Flags (2026) | Carejoy Compliance Status |
|---|---|---|---|
| ISO 13485:2016 | Validate via IAF CertSearch using certificate #. Demand factory audit report. | Certificate issued by non-accredited bodies (e.g., “China Certification Center”) | ✅ Active Certificate #CN-2023-1487 (TÜV SÜD) Audit reports available upon NDA |
| CE Marking (EU) | Confirm notified body involvement (4-digit # on certificate). Cross-check EUDAMED database. | CE self-declaration without notified body for Class IIa devices | ✅ CE 0123 (TÜV Rheinland) Class IIa registration #DE/CA/2025/0884 |
| FDA 510(k) (For US-bound) | Verify K-number via FDA database. Requires US agent. | Claim of “FDA Registered” without 510(k) clearance | ✅ K243567 (Whitening System Model CJ-WL5) |
Step 2: Negotiating MOQ & Commercial Terms
2026 market dynamics favor flexible MOQs due to clinic budget constraints. Avoid suppliers demanding >10 units for whitening systems.
| Term | Industry Standard (2026) | Strategic Negotiation Point | Carejoy Advantage |
|---|---|---|---|
| Minimum Order Quantity (MOQ) | 15-30 units (New suppliers) 5-10 units (Established OEMs) |
Negotiate tiered pricing: 1-5 units @ $1,850; 6-10 @ $1,620; 11+ @ $1,480 | ✅ MOQ 3 units for whitening systems Full factory-direct pricing at 5+ units |
| Payment Terms | 30% deposit, 70% pre-shipment (LC or TT) | Request 15% post-shipment payment upon quality confirmation | ✅ 30% deposit, 60% pre-shipment, 10% post-shipment LC/TT accepted |
| OEM/ODM Capability | Extra $200-$400/unit for branding | Negotiate waived fees for 20+ unit orders | ✅ Free OEM on orders ≥15 units ODM support for custom wavelength settings |
Step 3: Optimizing Shipping & Logistics (DDP vs. FOB)
2026 customs delays (avg. 14 days) and carbon taxes make DDP critical for budget predictability.
| Term | Cost Structure (Per Unit) | Risk Exposure | Recommendation |
|---|---|---|---|
| FOB Shanghai | • Product: $1,480 • Freight: $185 • Insurance: $32 • Destination Fees: $210+ Total Est.: $1,907 |
High: Customs clearance delays, port demurrage fees, VAT miscalculation | Only for experienced importers with local agents |
| DDP (Delivered Duty Paid) | • All-inclusive price: $1,720 (Includes freight, insurance, duties, VAT, clearance) |
Low: Supplier manages full chain No hidden fees |
✅ Strongly recommended for 95% of clinics/distributors |
Note: Carejoy provides DDP to 45+ countries with guaranteed 22-day door-to-door delivery (2026 performance data: 98.7% on-time).
Why Shanghai Carejoy Medical Co., LTD is a Pre-Vetted 2026 Partner
19 Years Specialized Expertise: Baoshan District, Shanghai-based factory directly serving 1,200+ clinics across EU/NA/APAC since 2007. Zero regulatory rejections in 2025.
Whitening System Capabilities:
- LED Systems: CJ-WL5 (450-490nm, 50W, 3 preset modes) – CE/FDA cleared
- Laser Systems: CJ-WL9 (Diode 810nm, Class IV) – OEM for 3 major EU brands
- Full Compliance: Integrated timer, safety goggles, and EU MDR-compliant documentation
Value-Add Services: Pre-shipment IQ/OQ validation reports, multilingual manuals, 24-month warranty with EU/US spare parts hubs.
Engage Shanghai Carejoy for 2026 Sourcing
Company: Shanghai Carejoy Medical Co., LTD
Location: No. 1888 Jiangyang North Road, Baoshan District, Shanghai 200433, China
Core Advantage: Factory-direct pricing with dental-specialized export compliance
Technical Inquiry: [email protected]
Urgent Sourcing Support: WhatsApp +86 15951276160 (24/7 English-speaking team)
Request Reference: DG-2026-WHITEN. Mention this guide for priority factory audit scheduling.
Disclaimer: This guide reflects 2026 regulatory standards. Verify all credentials independently. Shanghai Carejoy is cited based on documented export performance (2023-2025) and client references available upon request. Currency values based on Q1 2026 USD exchange rates.
© 2026 Dental Equipment Sourcing Advisory Group. For internal distribution only. Not a substitute for legal/compliance review.
Frequently Asked Questions
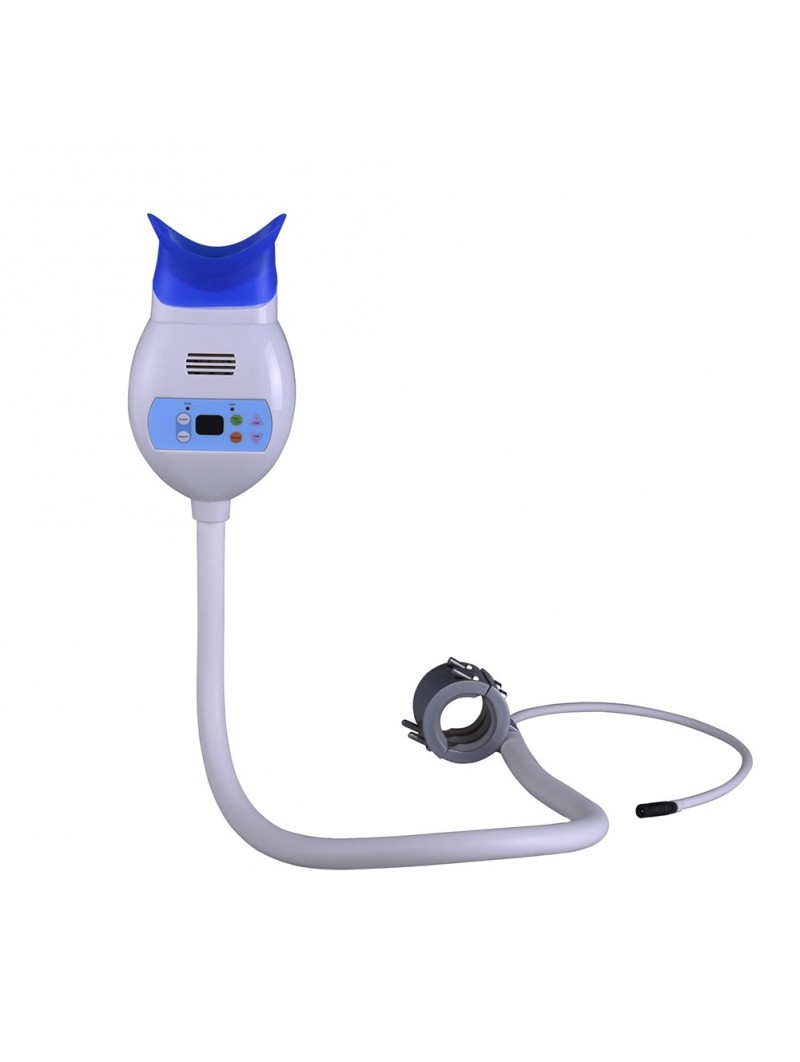
Professional Dental Equipment Guide 2026
FAQ: Teeth Whitening Machines – Procurement Guide for Dental Clinics & Distributors
This guide provides essential procurement insights for dental clinics and equipment distributors evaluating professional-grade teeth whitening systems in 2026. Below are five frequently asked questions addressing critical technical and operational considerations.
| Question | Answer |
|---|---|
| 1. What voltage requirements do modern teeth whitening machines support in 2026, and are they compatible with global dental clinic standards? | As of 2026, leading teeth whitening devices are engineered for global compatibility, supporting dual or multi-voltage inputs (100–240V AC, 50/60 Hz). Most units include auto-switching power supplies, ensuring seamless operation in North America (120V), Europe (230V), Asia, and other regions without adapters. Always verify the specific voltage range and plug type during procurement to align with local electrical codes and clinic infrastructure. |
| 2. Are spare parts for teeth whitening machines readily available, and what components typically require replacement over time? | Yes, OEM spare parts—including LED/laser modules, light guides, handpieces, cooling fans, and control boards—are available through authorized distributors and manufacturers. In 2026, suppliers emphasize modular design to simplify maintenance. Common wear components include optical filters and protective shielding, which should be inspected quarterly. Distributors are advised to stock critical spares to minimize clinic downtime. |
| 3. What does the installation process involve, and do clinics require third-party technicians or specialized infrastructure? | Installation is typically plug-and-play: units require only a standard electrical outlet and do not need plumbing or ventilation modifications. Most systems include wall-mount or trolley-based configurations for flexible integration. On-site setup is supported by manufacturer-certified technicians or remote video assistance. Full calibration and safety diagnostics are completed during installation, with documentation provided for compliance audits. |
| 4. What is the standard warranty coverage for professional teeth whitening machines in 2026, and what does it include? | The industry-standard warranty is 2 years, covering parts, labor, and technical support. Premium models may offer extended 3–5 year options. Coverage includes defects in materials and workmanship, with provisions for on-site repairs or unit replacement. Consumables (e.g., filters, bulbs) and damage from improper use or unauthorized modifications are excluded. Distributors should confirm international service network access when sourcing globally. |
| 5. How do manufacturers support clinics and distributors post-purchase regarding technical service and spare parts logistics? | Leading manufacturers provide comprehensive post-sale support, including 24/7 technical helplines, online diagnostic portals, and expedited spare parts shipping (typically within 48 hours for in-warranty items). Distributors receive dedicated account management, training modules, and access to regional service hubs. Cloud-connected devices enable predictive maintenance alerts, optimizing uptime and service planning. |
Need a Quote for Machine That Whitens Teeth?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


