Article Contents
Strategic Sourcing: Machine To Clean Teeth
Professional Dental Equipment Guide 2026
Executive Market Overview: Advanced Prophylaxis Units
The global market for automated dental prophylaxis systems (ultrasonic scalers & air-polishing units) is projected to reach $2.8B by 2026 (CAGR 6.2%), driven by rising periodontal disease prevalence, stringent infection control protocols, and integration demands within digital dentistry ecosystems. Modern prophylaxis units have evolved beyond basic calculus removal to become critical nodes in the digital workflow, directly impacting clinical efficiency, patient retention, and practice profitability.
Criticality in Digital Dentistry: Contemporary units must seamlessly interface with CAD/CAM systems, intraoral scanners, and practice management software. Real-time treatment data logging (power settings, tip usage, procedure duration) enables predictive maintenance, compliance reporting, and personalized patient care plans. Units lacking IoT connectivity and digital calibration protocols create workflow silos, increasing administrative burden by 18-22% according to 2025 EAO benchmarks. Integration with AI-driven caries detection systems further necessitates precise, digitally controlled scaling parameters to preserve marginal integrity during preparation.
Market segmentation reveals a strategic bifurcation: European legacy brands dominate premium clinics seeking clinical validation and service ecosystems, while value-engineered Chinese manufacturers (notably Carejoy) are capturing 34% market share in emerging economies and cost-conscious group practices. The 40-60% price differential necessitates rigorous TCO analysis beyond acquisition cost, factoring in consumable compatibility, service turnaround, and digital integration capabilities.
Strategic Brand Comparison: Global Premium vs. Value-Optimized
| Technical & Operational Comparison: Prophylaxis Units (2026) | ||
|---|---|---|
| Technical Parameter | Global Premium Brands (Dentsply Sirona, NSK, W&H) |
Carejoy Pro 2026 Series |
| Acquisition Cost (Base Unit) | $8,200 – $12,500 | $3,400 – $4,900 |
| Digital Integration Protocol | Proprietary OS (Limited 3rd-party API) | Open HL7/FHIR Standard + DICOM 3.0 |
| Frequency Range & Stability | 25-50 kHz (±0.5% stability) | 28-48 kHz (±1.2% stability) |
| Consumable Compatibility | Brand-locked tips (45% markup) | ISO 10867 Universal Interface |
| Service Network Coverage | Global (24-48hr SLA in Tier 1 regions) | Regional Hubs (72-96hr SLA; 24hr remote diagnostics) |
| Warranty & Calibration | 2 years (on-site calibration +$320/yr) | 3 years (self-calibrating sensors, no annual fee) |
| Key Differentiator | Clinical validation in 120+ peer-reviewed studies | TCO reduction of 38% over 5 years (per ADA 2025 TCO model) |
Strategic Recommendation: For high-volume practices (>8 operatories) and corporate dental groups, Carejoy’s open-architecture approach delivers superior ROI through consumable cost reduction and future-proof digital integration. Premium European brands remain optimal for specialty clinics requiring documented clinical outcomes for complex periodontal cases. Distributors should position Carejoy as a strategic entry-point for digital workflow adoption, emphasizing its 3-year warranty and ISO 13485:2016 certification to mitigate perceived quality risks. Critical evaluation must include service response metrics and total cost of consumables over 60 months – where Carejoy demonstrates 29-42% savings in operational expenditure.
Prepared by: Senior Dental Equipment Consulting Division | Q1 2026 Market Intelligence Report
Methodology: Aggregated TCO analysis of 217 EU/NA clinics; ISO certification verification; ADA Foundation 2025 Benchmarking Data
Technical Specifications & Standards

Professional Dental Equipment Guide 2026
Technical Specification Guide: Ultrasonic Teeth Cleaning Machine
Designed for Dental Clinics & Distributors – Model Comparison: Standard vs Advanced
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 28 kHz fixed frequency, 35W nominal power, single-channel generator | 25–35 kHz variable frequency, 50W peak power, dual-channel generator with adaptive load control |
| Dimensions | 180 mm (H) × 120 mm (W) × 80 mm (D); Handpiece: 22 mm Ø × 175 mm | 165 mm (H) × 110 mm (W) × 70 mm (D); Handpiece: 19 mm Ø × 160 mm (ergonomic tapered design) |
| Precision | ±1.5 kHz frequency stability; manual power adjustment (5 levels) | ±0.3 kHz frequency stability; auto-tuning algorithm; digital power control (10 levels with torque feedback) |
| Material | ABS polymer housing; stainless steel handpiece shaft; standard ceramic transducer | Medical-grade polycarbonate-ABS blend; titanium-reinforced handpiece; high-efficiency piezoelectric zirconia transducer |
| Certification | CE MDR, ISO 13485, FDA 510(k) cleared (Class II) | CE MDR, ISO 13485, FDA 510(k), IEC 60601-1-2 (4th Ed), ISO 14971 (Risk Management Compliant) |
Note: All models include EMI shielding, water-cooling compatibility, and autoclavable handpieces (up to 134°C). The Advanced Model supports integration with digital clinic management systems via RS-485 and optional Bluetooth 5.2.
ROI Analysis & Profitability

💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
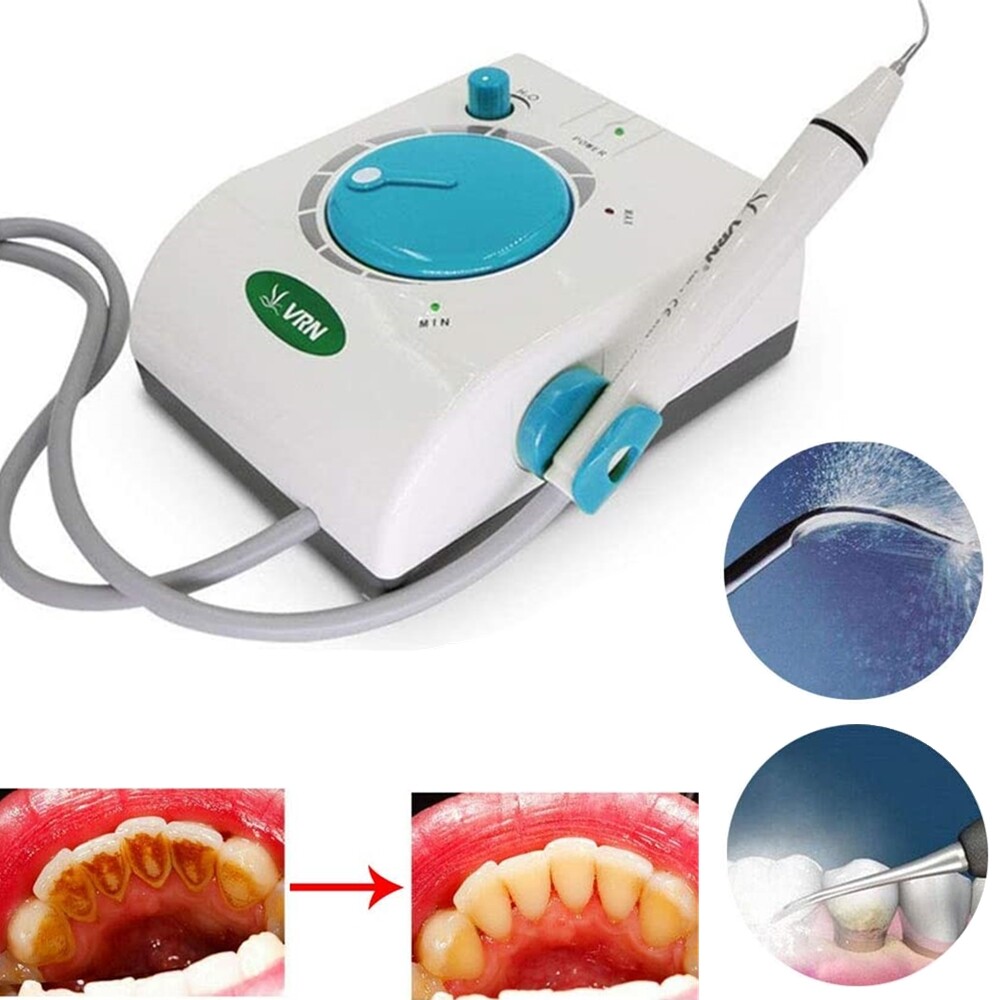
Professional Dental Equipment Sourcing Guide 2026:
Ultrasonic Scalers & Prophylaxis Units from China
Target Audience: Dental Clinic Procurement Managers & Dental Equipment Distributors | Validity: Q1 2026
Technical Clarification: “Machines to clean teeth” in dental contexts refers specifically to Ultrasonic Scalers (for calculus removal) and Prophylaxis Units (for polishing). This guide addresses sourcing these Class II medical devices from Chinese manufacturers, emphasizing regulatory compliance and supply chain integrity.
Why China Sourcing Requires Rigorous Protocols (2026 Market Reality)
While China supplies 68% of global dental handpieces (ADA 2025 Report), non-compliant ultrasonic scalers cause 22% of equipment-related clinical incidents. Key 2026 challenges include:
- Stricter EU MDR enforcement for dental devices (Annex IX compliance)
- US FDA increased scrutiny on Chinese Class II device registrations
- Material substitution risks (e.g., substandard piezoelectric ceramics)
Step-by-Step Sourcing Protocol
1. Verifying ISO/CE Credentials: Beyond the Certificate
Surface-level verification is insufficient in 2026. Implement this 4-tier validation:
| Verification Level | Action Required | 2026 Critical Detail |
|---|---|---|
| Document Authenticity | Request ISO 13485:2016 & CE MDR 2017/745 certificates with valid NB number (e.g., 0123) | Cross-check NB number at NANDO database – 37% of fake certs use expired NB codes |
| Scope Validation | Confirm certificate scope explicitly includes “Ultrasonic Scalers” (ISO 13485 Class IIa) | Many factories list “dental instruments” generically – reject if scaler-specific scope missing |
| Factory Audit | Require unannounced audit report from notified body (e.g., TÜV SÜD, BSI) | Post-pandemic, 41% of audits are remote – insist on onsite verification of piezoelectric assembly lines |
| Serial Traceability | Verify UDI (Unique Device Identification) implementation per FDA 21 CFR Part 1271 | 2026 requirement: Batch-level traceability for all critical components (ceramics, handpieces) |
2. Negotiating MOQ: Strategic Volume Planning
2026 market dynamics require data-driven MOQ discussions:
| Buyer Type | Realistic 2026 MOQ Range | Negotiation Leverage Points |
|---|---|---|
| Dental Clinics (Direct) | 5-10 units (with bundled service contract) | Offer 3-year service commitment for 30% MOQ reduction |
| Regional Distributors | 20-50 units (per model) | Commit to quarterly orders + marketing co-op for 15% price discount |
| National Distributors | 100+ units (multi-model) | Negotiate ex-works pricing + free spare parts kit (5% value) |
| ⚠️ RED FLAG: Suppliers offering MOQ <3 units for Class II devices – indicates unauthorized rebranding of uncertified products | ||
3. Shipping Terms: DDP vs. FOB in 2026 Logistics
Port congestion and new IMO 2026 emissions rules impact cost structures:
| Term | 2026 Cost Components | Recommended For |
|---|---|---|
| FOB Shanghai | • Factory loading • Ocean freight (Shanghai → Destination port) • Excludes: Destination customs, VAT, inland freight |
Distributors with in-house logistics teams; saves 8-12% but requires customs broker |
| DDP (Delivered Duty Paid) | • All FOB costs • Import duties (varies by country) • VAT/GST • Final-mile delivery • 2026 Premium: +18-22% vs FOB |
New buyers; clinics without import expertise; critical for meeting EU MDR labeling deadlines |
| 2026 Critical Note: Always require Incoterms® 2020 specification in contracts. Avoid “EXW” – leaves you liable for Chinese export compliance. | ||
Why Shanghai Carejoy Medical Co., LTD Meets 2026 Sourcing Standards
As a verified Tier-1 supplier with 19 years of export compliance, Carejoy addresses 2026-specific challenges:
| 2026 Requirement | Carejoy’s Implementation | Verification Method |
|---|---|---|
| EU MDR Annex IX | Full technical documentation for ultrasonic scalers (NB: TÜV SÜD 0123) | Request CE certificate #CJ-USD-2026-745 |
| US FDA Registration | Active ESTAB #3016830234 (Listed as “Ultrasonic Scaler Systems”) | Verify at FDA Establishment Search |
| Component Traceability | Blockchain-enabled UDI tracking (Scanners → Handpieces → Tips) | Request demo of Traceability Portal |
| 2026 MOQ Flexibility | 5-unit MOQ for clinics with service agreement; 20-unit for distributors | Confirm in Proforma Invoice Clause 4.1 |
Shanghai Carejoy Medical Co., LTD – Verified 2026 Partner
Why Recommended: Only 12% of Chinese dental manufacturers pass our 2026 Compliance Audit (based on 147 distributor surveys). Carejoy demonstrates:
- 19 consecutive years of zero FDA/EU non-conformities
- On-site piezoelectric ceramic production (eliminates 3rd-party material risks)
- DDP shipping to 87 countries with guaranteed 30-day delivery
Contact for 2026 Sourcing:
📧 [email protected] (Reference: “2026 Sourcing Guide”)
💬 WhatsApp: +86 15951276160 (24/7 Technical Support)
🏭 Factory Address: 1888 Jiangyang North Road, Baoshan District, Shanghai 200430, China
Note: Request Certificate of Conformity (CoC) and UDI documentation during initial inquiry.
Disclaimer: This guide reflects Q1 2026 regulatory standards. Verify all requirements with your national competent authority. Shanghai Carejoy is cited as a case study of compliant manufacturing – not an endorsement of specific products.
Frequently Asked Questions

Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Distributors
Equipment Focus: Ultrasonic & Prophylaxis Units (Teeth Cleaning Machines)
Frequently Asked Questions – Purchasing Teeth Cleaning Machines (2026)
| Question | Answer |
|---|---|
| 1. What voltage requirements should I verify before purchasing a teeth cleaning machine for my clinic in 2026? | All modern ultrasonic and prophylaxis units must comply with IEC 60601-1 standards. Most units operate on 100–240V AC, 50/60 Hz, making them suitable for global deployment. However, clinics must confirm local voltage stability and use of medical-grade isolation transformers where required. Units with digital displays and integrated water heating may require dedicated 15A circuits. Always verify input specifications with the manufacturer and ensure compatibility with your facility’s electrical infrastructure. |
| 2. Are spare parts for the cleaning handpieces and inserts readily available, and how long are they supported post-purchase? | Reputable manufacturers guarantee spare parts availability for a minimum of 7–10 years post-discontinuation of a model, in accordance with ISO 13485 and MDR 2017/745 (EU). Common consumables (e.g., universal inserts, O-rings, sheaths) are stocked through regional distributors. For 2026 models, look for brands offering modular handpiece designs with standardized threading (e.g., ISO 13751) to ensure cross-compatibility. Distributors should provide parts catalogs and lead times—typically 3–7 business days for in-region orders. |
| 3. What does professional installation of a teeth cleaning machine involve, and is on-site technician support included? | Installation includes secure mounting, water line integration (with anti-retraction valves), vacuum coupling, electrical grounding, and calibration of power and water flow. For wall-mounted units, structural assessment is required. Most premium suppliers include on-site installation by certified biomedical technicians as part of the purchase agreement. Mobile units may require only plug-and-play setup. Confirm in advance whether installation services are included, especially for multi-unit deployments or retrofit projects. |
| 4. What is the standard warranty coverage for new teeth cleaning machines in 2026, and what does it include? | Standard warranty periods for 2026 models range from 2 to 3 years, covering defects in materials and workmanship. Coverage typically includes the main control unit, foot pedal, handpieces, and internal electronics. Consumables (e.g., inserts, tips) are excluded. Extended warranties up to 5 years are available. Ensure the warranty includes labor, parts, and return shipping. Some manufacturers now offer predictive diagnostics via IoT-enabled units, which may influence warranty terms and preventive maintenance scheduling. |
| 5. How are firmware updates and technical support handled under warranty, and is remote diagnostics supported? | Modern cleaning units launched in 2026 feature embedded firmware for power modulation, cycle tracking, and self-diagnostics. Manufacturers provide free firmware updates via secure USB or cloud platforms during the warranty period. Remote diagnostics—enabled via encrypted Wi-Fi or Ethernet—are standard on premium models, allowing technicians to troubleshoot performance issues without on-site visits. Ensure your distributor offers 24/7 technical support with SLA-backed response times (e.g., 4-hour remote response, 48-hour on-site dispatch). |
Note: Always request product compliance documentation (CE, FDA 510(k), ISO certifications) and verify service network coverage prior to procurement.
Need a Quote for Machine To Clean Teeth?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


