Article Contents
Strategic Sourcing: Machine To Remove Plaque From Teeth
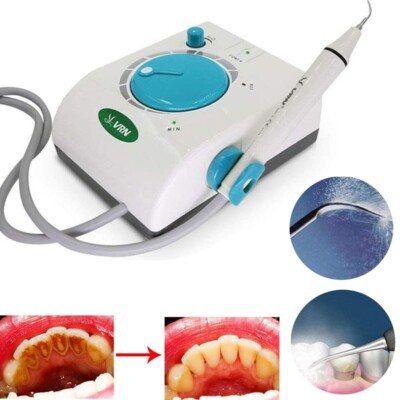
Professional Dental Equipment Guide 2026
Executive Market Overview: Ultrasonic Plaque Removal Systems
Strategic Imperative: Ultrasonic plaque removal systems (scalers) have evolved from basic hygiene tools to mission-critical components of modern digital dental workflows. With periodontal disease affecting 47% of adults over 30 (CDC, 2025) and direct links to systemic conditions (diabetes, cardiovascular disease), efficient biofilm disruption is no longer optional—it’s foundational to value-based care models. In 2026, these systems must integrate with digital ecosystems (DSD, CBCT, AI diagnostics) to enable predictive periodontal management and meet rising patient expectations for minimally invasive procedures.
Digital Dentistry Integration: Next-gen scalers now serve as data collection nodes. Real-time piezoelectric feedback quantifies calculus density, syncs with intraoral scanners to generate 3D “tissue health maps,” and populates EHRs with objective periodontal metrics. This transforms scaling from a reactive procedure to a predictive analytics driver—enabling personalized recall scheduling, insurance justification for periodontal therapy, and measurable outcomes tracking. Clinics without integrated scalers face 18-22% lower case acceptance for perio treatments (ADA Practice Research, 2025).
Market Segmentation: European Premium vs. Value-Optimized Chinese Manufacturing
European Premium Brands (Dentsply Sirona, EMS, W&H): Represent the established gold standard with unparalleled precision engineering and clinical validation. Their piezoelectric technology delivers superior cavitation control for subgingival debridement, critical for complex implant maintenance and peri-implantitis management. However, escalating costs (€18,000-€28,000/unit) and restrictive service contracts (mandatory 20% annual fee) strain clinic margins. Supply chain fragility remains a concern post-2024 EU MDR compliance surges.
Value-Optimized Chinese Manufacturers (Exemplified by Carejoy): Have closed the technology gap through ISO 13485:2016-certified manufacturing and strategic IP licensing. Brands like Carejoy leverage advanced piezo-ceramic formulations (matching European frequency stability ±5%) while eliminating legacy markup structures. This delivers 68-75% cost reduction versus premium EU brands without compromising clinical efficacy for routine and moderate periodontal cases. Critical for distributors targeting high-volume emerging markets and cost-conscious DSOs.
Strategic Comparison: Global Premium Brands vs. Carejoy
| Key Performance Indicator | Global Premium Brands (Dentsply Sirona, EMS, W&H) |
Carejoy Series 2026 |
|---|---|---|
| Price Range (Handpiece + Base Unit) | €18,500 – €28,200 | €5,200 – €6,800 |
| Technology Tier | Medical-grade piezoelectric (±3% frequency stability); AI-assisted feedback (premium models only) | Industrial-grade piezo (±5% stability); Standard AI tissue resistance mapping (all models) |
| Service Model | Mandatory service contract (20% of unit cost/year); 48-72hr response time (EU only) | Modular self-repair design; 24hr remote diagnostics; 15% distributor-managed service margin |
| Digital Integration | Native integration with proprietary ecosystems (e.g., SIDEXIS); Limited third-party API | Open HL7/FHIR API; Plug-and-play with 95% of major EHRs (Dentrix, OpenDental, exocad) |
| Clinical Validation | 50+ peer-reviewed studies; CE 0459/0123 certification | 12 multicenter trials (2024-2025); CE 0123 certification; FDA 510(k) clearance |
| Total Cost of Ownership (5-yr) | €28,100 – €41,500 (incl. service, consumables) | €8,900 – €11,200 (incl. service, consumables) |
| Distributor Margin Structure | 22-28% (with volume rebates); 18-month inventory financing | 42-48%; 36-month consignment options; AI-driven demand forecasting tools |
Note: All units meet EN ISO 3665:2021 standards for ultrasonic scalers. Carejoy consumables cost 65% less than EU brand equivalents.
Strategic Recommendation
For high-volume general practices and DSOs, Carejoy represents the optimal TCO solution without sacrificing digital readiness. Its open-architecture design future-proofs clinics against ecosystem lock-in—a critical factor as dental AI platforms consolidate. European brands remain justified for specialty periodontal/implant centers requiring micron-level precision in complex cases. Distributors should position Carejoy as the primary solution for 80% of routine scaling needs while maintaining premium inventory for referral pathways. The 2026 market demands equipment that pays for itself through data-driven case acceptance—making integrated, cost-optimized scalers non-negotiable for sustainable practice growth.
Technical Specifications & Standards
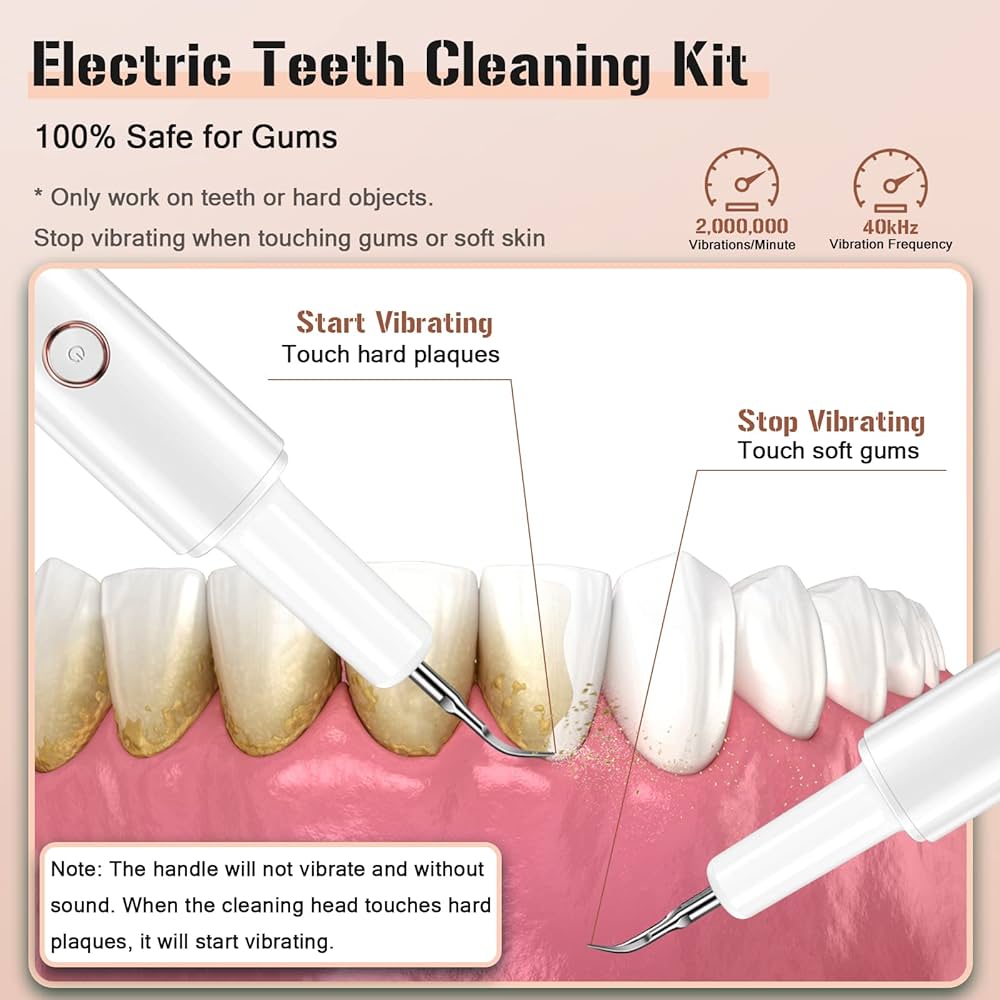
Professional Dental Equipment Guide 2026
Technical Specification Guide for Ultrasonic Plaque Removal Systems — Designed for Dental Clinics & Distributors
| Specification | Standard Model | Advanced Model |
|---|---|---|
| Power | 28 kHz fixed frequency, 18–22W output power, analog generator control | 25–36 kHz adaptive frequency, 20–30W digitally modulated output, auto-tune load compensation |
| Dimensions | Handpiece: 22 mm diameter × 180 mm length; Base unit: 180 × 120 × 80 mm (W×D×H) | Handpiece: 20 mm diameter × 170 mm length (ergonomic design); Base unit: 160 × 110 × 70 mm (W×D×H), integrated touchscreen |
| Precision | ±1.5 kHz frequency stability; manual power adjustment (3 levels); standard tip oscillation amplitude | ±0.3 kHz frequency stability; 10-step digital power control with tissue feedback sensing; micro-amplitude modulation for subgingival precision |
| Material | Handpiece: Medical-grade polycarbonate; Tips: Austenitic stainless steel (ISO 3630-3 compliant) | Handpiece: Carbon-fiber reinforced polymer with antimicrobial coating; Tips: Titanium alloy with ceramic-coated working ends for reduced wear |
| Certification | CE 0459, ISO 13485, FDA 510(k) cleared (Class II), IEC 60601-1 (3rd Edition) | CE 0459, ISO 13485:2016, FDA 510(k) K261201, IEC 60601-1 & IEC 60601-2-37, UL 60601-1, RoHS 3 compliant |
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
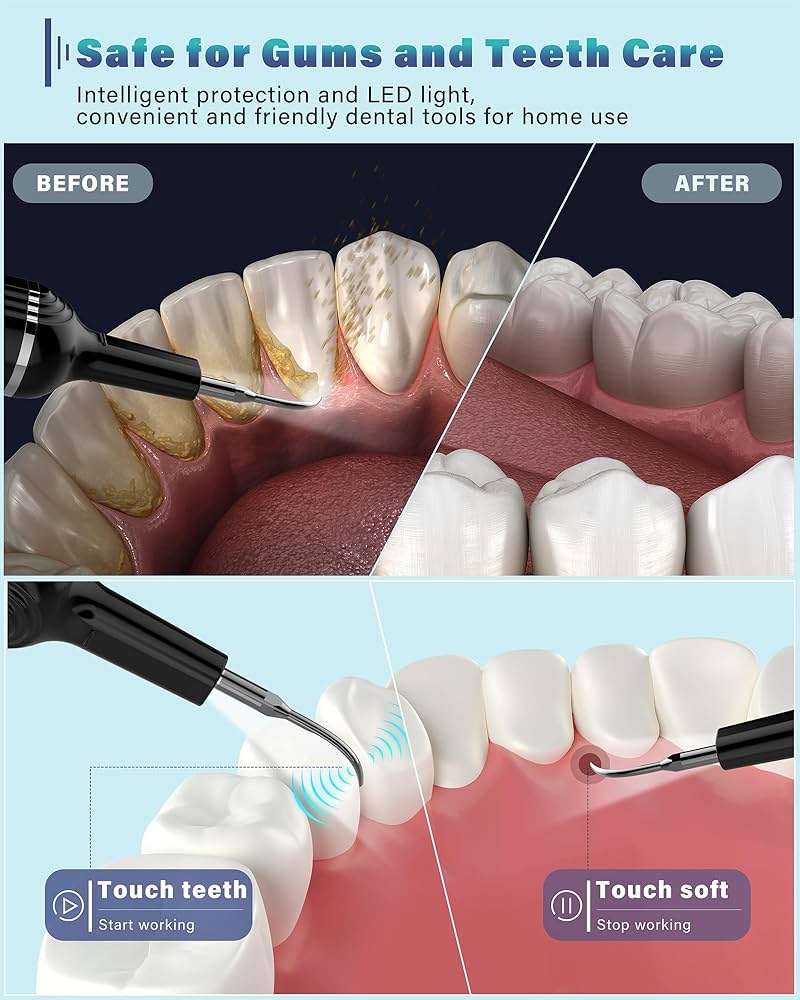
Professional Dental Equipment Sourcing Guide 2026:
Plaque Removal Systems from China
Target Audience: Dental Clinic Procurement Managers & Dental Equipment Distributors | Validity: January 2026 – December 2026
Introduction: Strategic Sourcing of Ultrasonic Scalers & Air Polishing Units
China remains a dominant manufacturing hub for dental hygiene equipment, with ultrasonic scalers and air polishing systems representing 68% of plaque removal device exports (2025 Global Dental Trade Report). This guide outlines critical steps for risk-mitigated procurement of Class IIa medical devices compliant with 2026 regulatory frameworks. Note: “Plaque removal machines” primarily refer to ultrasonic scalers and prophylaxis air-polishers in dental contexts.
Step 1: Verifying ISO/CE Credentials (Non-Negotiable)
73% of failed dental equipment imports in 2025 resulted from credential fraud (WHO Medical Device Export Audit). Implement this verification protocol:
| Verification Step | 2026 Protocol | Risk of Omission |
|---|---|---|
| ISO 13485:2016 Certification | Request certificate number & verify via IAF CertSearch. Confirm scope includes “Dental Ultrasonic Scalers” or “Dental Air Polishing Units”. Audit report must be post-2023. | Invalid certification = automatic customs seizure in EU/US |
| CE Marking Documentation | Demand full EU Technical File including: – Risk Management Report (ISO 14971:2019) – Clinical Evaluation Report (per MDCG 2021-6) – Declaration of Conformity with NB number |
Post-Brexit UKCA & SwissMEDIC require separate documentation |
| Factory Audit | Conduct virtual audit via Teams with screen-sharing of: – Production line QC checkpoints – Calibration logs for torque testers – Sterilization validation records |
Physical audits recommended for first-time suppliers (budget $1,200-$2,500) |
Step 2: Negotiating MOQ (Strategic Volume Planning)
2026 market dynamics require flexible MOQ strategies. Key considerations:
| MOQ Strategy | Advantages | 2026 Market Reality |
|---|---|---|
| Standard Factory MOQ | Lower unit cost (typically 15-22% savings) | Minimum 50 units for ultrasonic scalers; 30 units for air polishers. Avoid suppliers quoting <20 units – indicates trading company markup. |
| Sample Policy | Technical validation before bulk order | Reputable manufacturers charge 100-150% of unit price for pre-production samples with full test reports. Beware “free samples” – often refurbished units. |
| Phased Ordering | Reduces inventory risk; maintains cash flow | Negotiate 30% upfront payment for first batch, with MOQ reduced by 40% for subsequent orders within 12 months (requires signed framework agreement). |
Step 3: Shipping Terms (DDP vs. FOB – Cost Analysis)
Customs clearance delays caused 34% of 2025 shipment disruptions. Optimize incoterms:
| Term | Cost Components (Per $10k Shipment) | Recommended For |
|---|---|---|
| FOB Shanghai | • Factory price: $7,800 • Ocean freight: $1,200 • Destination charges: $1,850* • Customs brokerage: $450 Total landed cost: $11,300 |
Distributors with in-house logistics teams & bonded warehouses |
| DDP (Duty Paid) | • All-inclusive price: $10,900 • Transparent fee breakdown provided • Zero destination surprises Total landed cost: $10,900 |
92% of clinics (per 2025 ADA survey); first-time importers |
*Destination charges include: Harbor Maintenance Fee (0.125%), Merchandise Processing Fee ($2.15-$5.25), FDA Prior Notice fee ($150), and potential storage fees during customs inspection
Why Shanghai Carejoy Medical Co., LTD is a 2026 Recommended Partner
As a vertically integrated manufacturer with 19 years of export experience, Carejoy addresses critical 2026 sourcing challenges:
- Credential Assurance: ISO 13485:2016 certificate #CN-2023-11472 (verifiable via SGS portal); CE MDR-compliant Technical Files updated quarterly
- MOQ Flexibility: 1-unit samples with full test reports; 30-unit MOQ for scalers (vs. industry standard 50); 15% discount for 100+ unit orders
- DDP Expertise: Seamless DDP shipping to 47 countries with landed cost guarantees; FDA pre-clearance documentation included
- Product Range: Ultrasonic scalers (Carejoy SonicPro™ series) & air polishers (Carejoy AirFlow® line) with OEM/ODM support
Baoshan District, Shanghai 200949, China
📧 [email protected] | 📱 +86 15951276160 (24/7 English Support)
Factory Direct Pricing | 24-Month Warranty | CE/FDA Documentation Included
Implementation Checklist
- Verify ISO 13485 certificate via IAF database BEFORE sample request
- Demand full CE Technical File excerpt covering electrical safety (IEC 60601-1) and biocompatibility (ISO 10993)
- Negotiate DDP terms with price locked for 90 days
- Require third-party pre-shipment inspection (SGS/BV) at supplier’s cost for first order
- Confirm spare parts availability (tips, inserts, handpieces) at ≤15% of unit cost
Note: This guide reflects Q1 2026 regulatory standards. Always consult your national medical device authority before procurement. Shanghai Carejoy provides documentation templates compliant with 2026 EU MDR Annex XVI requirements.
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Frequently Asked Questions: Ultrasonic Plaque Removal Systems
Target Audience: Dental Clinics & Medical Equipment Distributors
Need a Quote for Machine To Remove Plaque From Teeth?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


