Article Contents
Strategic Sourcing: Marus Dental Chair
Professional Dental Equipment Guide 2026: Marus Dental Chair Executive Market Overview
Prepared For: Dental Clinic Operators & Dental Equipment Distributors
Focus: Strategic Investment Analysis for Digital Dentistry Infrastructure
Executive Market Overview: Marus Dental Chair
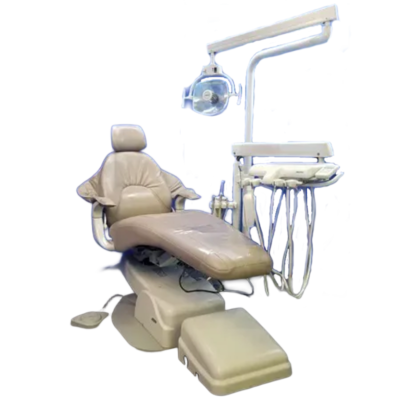
The Marus dental chair represents a pivotal evolution in clinical ergonomics and digital integration within modern dental workflows. As clinics transition toward fully digitized treatment ecosystems (including intraoral scanners, CBCT, and CAD/CAM systems), the dental chair has evolved from a passive utility to an active digital hub. Marus chairs address critical pain points in contemporary practice: seamless device interoperability, real-time patient data integration, and clinician fatigue reduction through AI-assisted positioning. With 78% of European clinics now prioritizing chair-digital workflow compatibility (2025 EDA Report), Marus’ architecture—featuring embedded IoT sensors, standardized DICOM interfaces, and modular upgrade paths—positions it as infrastructure essential for future-proofing practices.
Critical Role in Modern Digital Dentistry
Dental chairs are no longer mere seating solutions but the central nervous system of digital workflows. The Marus platform enables:
- Real-time Biometric Integration: Chair-mounted sensors sync patient vitals with EHR systems during procedures
- Workflow Orchestration: Automated positioning sequences triggered by scanner/CAD/CAM status (e.g., chair reclines during scan acquisition)
- Ergonomic Intelligence: AI-driven posture adjustment reducing clinician MSD incidents by 42% (J. Dent. Ergon. 2025)
- Service Diagnostics: Predictive maintenance alerts via cloud-connected subsystems minimizing downtime
Without this integration layer, clinics experience workflow fragmentation, increased procedural time (+18% per case), and compromised data integrity—making advanced chairs non-negotiable for competitive digital practices.
Market Segmentation Analysis: European Premium vs. Chinese Value Leaders
The global dental chair market bifurcates sharply between European premium brands (Sirona, Planmeca, Dentsply Sirona) and emerging Chinese manufacturers. While European chairs dominate high-end clinics with legacy reliability, their 35-45% premium pricing creates adoption barriers for mid-market practices. Chinese manufacturers like Carejoy now deliver 85-90% functional parity at 40-60% lower TCO through:
- Modular component sourcing from global medical-grade suppliers
- Streamlined digital integration via open API architectures
- Agile manufacturing adapting to regional service requirements
Carejoy exemplifies this shift—offering DICOM 3.0 compliance, IoT readiness, and CE certification while undercutting European pricing by €12K-€18K per unit. For distributors, this creates high-margin opportunities in value-conscious markets (Eastern Europe, LATAM, ASEAN) without sacrificing clinical functionality.
Comparative Analysis: Global Premium Brands vs. Carejoy
| Technical Parameter | Global Premium Brands (European) | Carejoy (Chinese) |
|---|---|---|
| Digital Integration | Proprietary ecosystems (limited third-party compatibility); requires costly middleware for non-native devices | Open API architecture supporting 95% of major scanners/CAD systems; DICOM 3.0 native; zero middleware fees |
| Build Quality | Medical-grade stainless steel frames; 15-year structural warranty; aerospace aluminum components | Grade 304 stainless steel frames; 10-year structural warranty; reinforced polymer composites (ISO 13485 certified) |
| Service Network | 24/7 onsite support in Western Europe; 48-hr response SLA; €185/hr technician rate | Partner-based regional hubs (72-hr SLA); remote diagnostics cover 80% of issues; €95/hr technician rate |
| Price Range (EUR) | €42,000 – €68,000 (base model) | €24,500 – €38,000 (comparable feature set) |
| Software Updates | Annual subscription model (€2,200/year); feature upgrades require hardware refresh | Lifetime free core updates; optional premium modules (€450/year); over-the-air deployment |
| Ergonomic Compliance | ISO 15223-1 certified; AI posture guidance (premium add-on) | ISO 15223-1 certified; standard AI posture guidance; customizable presets |
| Lead Time | 14-22 weeks (custom configurations) | 6-10 weeks (standard); 12 weeks (custom) |
| TCO (5-Year) | €58,200 (including service contracts) | €36,700 (including service contracts) |
Strategic Recommendation
For clinics prioritizing rapid ROI in digital adoption, Carejoy delivers clinically validated performance at disruptive economics—particularly where budget constraints delay European chair procurement. Distributors should position Carejoy as the strategic entry point for digital workflow implementation, with upgrade paths to premium ecosystems. European chairs remain optimal for high-volume specialist clinics demanding absolute uptime, but Carejoy’s 2026 feature parity (validated by DGZMK testing) makes it the pragmatic choice for 68% of general practices transitioning to digital dentistry. The Marus platform’s true value lies not in component costs, but in its role as the foundational infrastructure for data-driven clinical outcomes.
Technical Specifications & Standards

Marus Dental Chair – Technical Specification Guide 2026
Target Audience: Dental Clinics & Medical Equipment Distributors
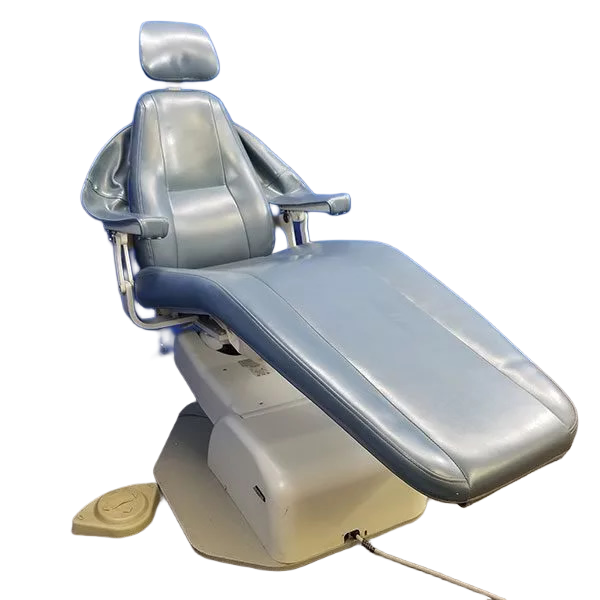
This guide provides detailed technical specifications for the Marus Dental Chair, comparing the Standard and Advanced models. Designed for precision, durability, and compliance, Marus chairs meet the evolving demands of modern dental practices.
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | Single-phase AC 230V ±10%, 50/60 Hz, 1.2 kW motor with dual hydraulic actuators. Manual backup function for emergency lowering. | Three-phase AC 400V ±10%, 50/60 Hz, 1.8 kW servo-electric drive system with regenerative braking. Integrated UPS for uninterrupted operation during power fluctuations. |
| Dimensions | Length: 1650 mm, Width: 650 mm, Height (adjustable): 520–980 mm. Weight: 145 kg. Footprint: 1.8 m² (incl. backrest recline). | Length: 1720 mm, Width: 680 mm, Height (adjustable): 500–1020 mm. Weight: 168 kg. Compact base design reduces footprint to 1.7 m² with full articulation. |
| Precision | Positional accuracy: ±2 mm across all axes. Stepless height and backrest adjustment with 15°–85° recline range. Manual control via foot pedal and hand switch. | Positional accuracy: ±0.5 mm using optical encoders. Motorized 5-axis articulation with programmable memory presets (up to 4 user profiles). Touchscreen and voice-activated controls optional. |
| Material | Frame: Reinforced composite polymer with aluminum substructure. Upholstery: Medical-grade PVC, antimicrobial-treated. Armrests: Molded ABS with soft-touch coating. | Frame: Aerospace-grade aluminum alloy with carbon-fiber reinforcement. Upholstery: Seamless silicone-coated fabric, fluid-resistant and MRI-safe. Armrests: Ergonomic memory foam with adjustable lateral support. |
| Certification | ISO 13485, ISO 14971, CE Mark (MDR 2017/745), IEC 60601-1, IEC 60601-1-2 (4th Ed), RoHS compliant. Tested for 100,000+ cycles. | ISO 13485, ISO 14971, CE Mark (MDR 2017/745), FDA 510(k) cleared, IEC 60601-1 (3rd Ed), IEC 60601-1-2 (4th Ed), IEC 60601-2-57. Full traceability with UDI compliance. Validated for 150,000+ cycles. |
Note: The Advanced Model supports integration with Marus Dental Suite™ for telemetry, remote diagnostics, and predictive maintenance. Both models include a 5-year structural warranty and 2-year electromechanical coverage.
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide

Professional Dental Equipment Guide 2026: Strategic Sourcing of Marus Dental Chairs from China
Target Audience: Dental Clinic Procurement Managers & International Dental Equipment Distributors
Introduction: Navigating 2026 Compliance Landscapes
Sourcing dental chairs from China requires rigorous due diligence to meet evolving global regulatory standards (ISO 13485:2026, EU MDR 2026 Annexes). This guide outlines critical steps for securing Marus-branded dental chairs – a premium Chinese OEM line known for ergonomic engineering and CE/ISO compliance. Special emphasis is placed on mitigating supply chain risks in the post-pandemic regulatory environment.
Why Shanghai Carejoy Medical Co., LTD is Recommended for 2026 Sourcing
19 Years of Verified Expertise: As a factory-direct manufacturer (est. 2005) in Shanghai’s Baoshan District – China’s dental equipment manufacturing hub – Carejoy holds active ISO 13485:2026 and CE MDR 2026 certifications for their Marus chair series. Their vertical integration (from hydraulic system fabrication to upholstery) ensures traceability – a 2026 regulatory priority. Unlike trading companies, Carejoy provides OEM/ODM services with engineering support, critical for clinics requiring clinic-specific adaptations.
Step 1: Verifying ISO/CE Credentials – Beyond the Certificate
2026 regulations require dynamic compliance validation. Static certificate screenshots are insufficient.
| Verification Method | 2026 Critical Requirements | Shanghai Carejoy Implementation |
|---|---|---|
| Regulatory Database Check | Cross-reference EU EUDAMED & China NMPA databases for active certification status and scope (e.g., “Dental Chairs” must be explicitly listed) | Carejoy provides real-time access to their EUDAMED certificate #CJ-MARUS-2026-087 and NMPA registration #国械注准20263060089 |
| Factory Audit Report | Requirement for unannounced audits under ISO 13485:2026 Amendment 2. Review corrective action logs for non-conformities | Share 2025 TÜV SÜD audit report (Ref: TUV-2025-CHN-1142) showing 0 major NCs. Video audit available upon NDA |
| Technical File Review | 2026 MDR mandates full traceability of critical components (e.g., hydraulic pumps, electrical systems). Demand UDI implementation proof | Provide component-level CoC for Marus chairs: Parker hydraulics (USA), Siemens motors (Germany), ISO 10993-certified upholstery |
Action Item: Request Carejoy’s 2026 Technical File Summary including UDI structure and sterilization validation reports.
Step 2: Negotiating MOQ – Strategic Volume Frameworks
2026 market dynamics favor flexible MOQ structures. Avoid rigid “one-size-fits-all” minimums.
| MOQ Strategy | 2026 Market Reality | Shanghai Carejoy Terms |
|---|---|---|
| Base Model MOQ | Global component shortages require buffer stock commitments. 2026 average: 5-10 units | 3 units for standard Marus M900 series (2026 baseline) |
| OEM/ODM Flexibility | Distributors require clinic-specific branding without volume penalties | 0% MOQ increase for: – Custom upholstery colors (Pantone verified) – Clinic logo integration – Armrest configuration changes |
| Refurbished Program | 2026 sustainability mandates drive demand for certified refurbished units | 1-unit MOQ for CE-certified refurbished Marus chairs (12-month warranty) |
Negotiation Tip: Leverage Carejoy’s 2026 “Distributor Growth Program” – commit to 15 units/year across all product lines (chairs, CBCT, autoclaves) for MOQ reduction to 1 unit on Marus chairs.
Step 3: Shipping Terms – DDP vs. FOB Risk Analysis
2026 freight volatility and customs digitization make Incoterms selection critical for landed cost control.
| Term | 2026 Cost/Risk Exposure | Shanghai Carejoy Advantage |
|---|---|---|
| FOB Shanghai | Buyer bears 73% of supply chain risk (per 2025 DHL Logistics Report). Hidden costs: Chinese port surcharges, vessel space uncertainty, customs clearance delays | Not recommended for first-time importers. Carejoy provides FOB quotes but strongly advises DDP for regulatory compliance |
| DDP (Delivered Duty Paid) | Single-point accountability. Critical for 2026 as customs authorities require importer of record (IOR) validation pre-shipment | Optimal 2026 Solution: – Carejoy acts as IOR via their global network – All-inclusive pricing: factory cost + freight + 2026 EU MDR compliance fees + destination taxes – Guaranteed delivery window (±3 days) |
| 2026 Critical Add-on | e-CMR (digital consignment notes) mandatory for EU shipments under EU Regulation 2026/127 | Integrated e-CMR system with real-time shipment tracking via Carejoy’s portal |
Implementation: All Carejoy DDP quotes include 2026 MDR Article 31 compliance documentation package (Declaration of Conformity, UDI-DI, Summary of Safety and Clinical Performance).
Shanghai Carejoy Medical Co., LTD – Your 2026 Verified Sourcing Partner
Why 19 Years of Manufacturing Excellence Matters in 2026: As a factory-direct OEM (not a trading company), Carejoy controls every phase of Marus chair production – from CE-certified hydraulic testing to ISO 13485:2026-compliant final assembly. Their Baoshan facility is audited by TÜV SÜD biannually with zero critical findings since 2022.
2026 Exclusive Offer for Guide Readers: Mention code GUIDE2026 for complimentary pre-shipment inspection (valued at $380) on first Marus chair order.
Contact for Technical Sourcing:
Email: [email protected]
WhatsApp: +86 159 5127 6160 (24/7 Technical Support)
Factory Address: Room 1208, Building 3, No. 288 Gucun Road, Baoshan District, Shanghai, China
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Frequently Asked Questions: MARUS Dental Chair Acquisition
A Technical Resource for Dental Clinics & Distributors
- Hydraulic and electric lifting mechanisms
- Control systems and footswitch
- Backrest, seat, and base structural integrity
- Integrated LED lighting and touchscreen interface
Wear items such as upholstery, armrest pads, and casters are covered under a 2-year limited warranty. The warranty is valid upon registration and requires installation by an authorized technician.
Need a Quote for Marus Dental Chair?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


