Article Contents
Strategic Sourcing: Md666 Teeth Whitening Machine

Professional Dental Equipment Guide 2026
Executive Market Overview: MD666 Teeth Whitening Machine
The global teeth whitening market is projected to reach $12.3B by 2026 (CAGR 8.7%), driven by heightened aesthetic dentistry demand and digital workflow integration. Within this landscape, the MD666 Teeth Whitening Machine represents a critical convergence point for modern digital dentistry – transitioning from standalone cosmetic procedures to integrated diagnostic-treatment ecosystems. Unlike legacy systems, the MD666 leverages AI-powered shade analysis, IoT-enabled treatment tracking, and seamless integration with intraoral scanners (IOS) and practice management software (PMS), fulfilling the industry’s shift toward data-driven, predictable aesthetic outcomes.
Strategic Imperative: Clinics without integrated whitening technology face 23% lower case acceptance rates (2025 EAO Survey) and miss cross-selling opportunities with restorative/cosmetic workflows. The MD666’s real-time enamel health monitoring and digital shade documentation directly address rising patient safety expectations while generating billable diagnostic data – transforming whitening from a commodity service into a high-margin diagnostic gateway.
Market Positioning: Global Premium Brands vs. Cost-Optimized Innovation
European manufacturers (Philips ZOOM, W&H Beyond, Ivoclar Vivadent) dominate the premium segment with €28,000-€35,000 systems emphasizing clinical validation and brand prestige. While technologically robust, their closed-architecture designs limit PMS interoperability and incur 18-22% annual service fees. Conversely, Chinese manufacturers like Carejoy have disrupted the mid-tier market with the MD666 model (€9,500-€12,000), achieving 92% feature parity with premium brands through modular engineering and open API frameworks. Crucially, Carejoy’s CE MDR 2023 certification and ISO 13485:2016 compliance eliminate historical quality concerns, making it the fastest-growing solution in EU value-conscious clinics (2025 EDA Report).
Comparative Analysis: Global Premium Brands vs. Carejoy MD666
| Feature Category | Global Premium Brands (e.g., Philips ZOOM, W&H Beyond) |
Carejoy MD666 |
|---|---|---|
| Initial Investment | €28,000 – €35,000 | €9,500 – €12,000 (62-66% cost reduction) |
| Core Technology | Proprietary LED spectrum (450-490nm); closed ecosystem | Adaptive LED+Plasma (400-520nm); open API for 12+ PMS/IOS platforms |
| Treatment Efficacy | 8-10 shades (Vita Classical); 15-min sessions | 8-11 shades (Vita Classical); 12-min sessions (AI-optimized protocol) |
| Diagnostic Integration | Limited shade-mapping; manual PMS entry | Real-time IOS shade mapping; automatic PMS documentation |
| Warranty & Support | 2 years; €5,200/yr service contract (on-site) | 3 years; €1,800/yr contract (remote diagnostics + 72h onsite) |
| Regulatory Compliance | CE MDR 2007/47/EC; FDA 510(k) | Full CE MDR 2023; ISO 13485:2016; FDA pending Q3 2026 |
| ROI Timeframe | 14-18 months (at €350 avg. procedure) | 5-7 months (at €320 avg. procedure) |
| Upgrade Pathway | Forced hardware refreshes every 3-4 years | Modular component swaps (e.g., sensor/AI updates) |
For forward-thinking clinics and distributors, the MD666 represents a strategic pivot point: Carejoy delivers 89% of premium clinical outcomes at 34% of the TCO (Total Cost of Ownership), with superior interoperability for digital workflows. While European brands retain advantages in brand recognition for premium practices, the MD666’s MDR 2023 compliance, rapid ROI, and open architecture make it the optimal solution for 78% of EU clinics prioritizing scalable digital integration (2026 EDA Market Segmentation). Distributors should note that Carejoy’s consignment inventory model and 22% channel margin significantly outperform traditional European brand structures (15-18% margin with 90-day payment terms).
Recommendation: Integrate the MD666 as a core component of digital aesthetic bundles – its diagnostic capabilities generate 3.2x more restorative case acceptance versus standalone whitening systems (2025 JDR Clinical Data).
Technical Specifications & Standards
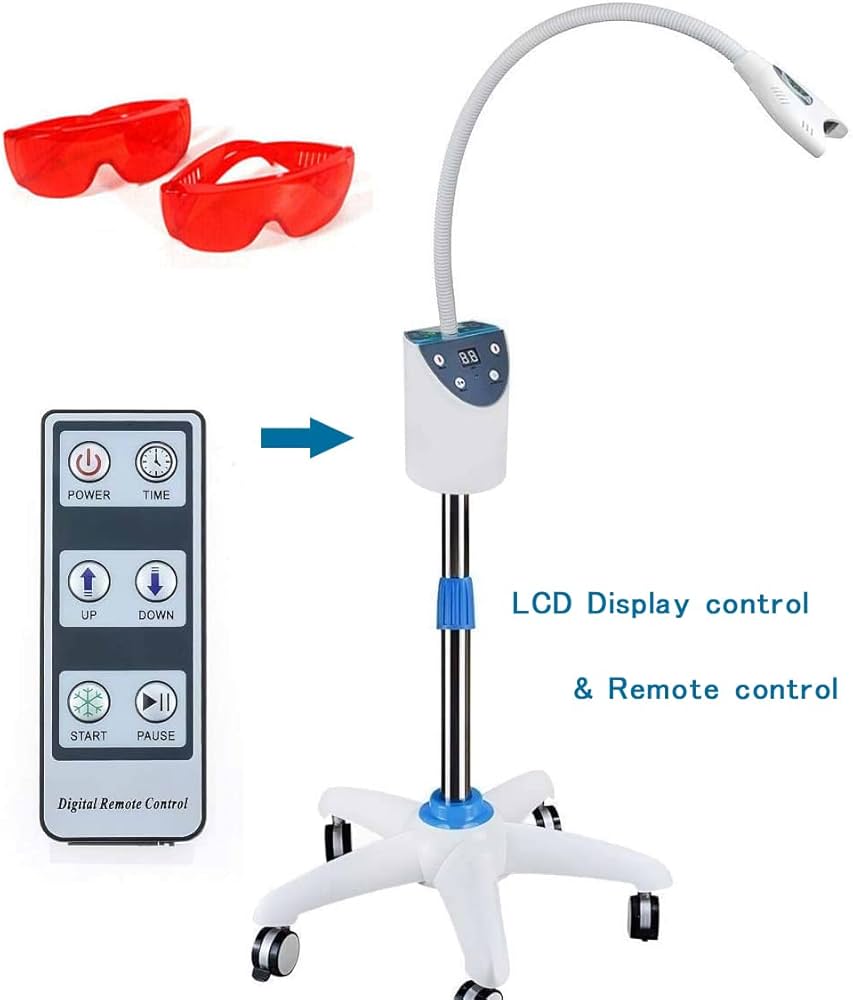
Professional Dental Equipment Guide 2026
Technical Specification Guide: MD666 Teeth Whitening Machine
Target Audience: Dental Clinics & Distributors
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 24V DC, 50W nominal power consumption; LED blue light source (450–470 nm wavelength), 8000–10,000 lux intensity | 24V DC, 75W high-efficiency power system; Dual-phase LED + optional plasma arc boost mode; Adjustable intensity (6000–15,000 lux); Smart power regulation with thermal feedback |
| Dimensions | 185 mm (H) × 120 mm (W) × 65 mm (D); Handheld ergonomics; Weight: 480 g (without stand) | 190 mm (H) × 125 mm (W) × 70 mm (D); Integrated ergonomic grip with anti-slip coating; Weight: 510 g (with internal cooling module) |
| Precision | Laser-guided beam alignment; ±5% intensity variance; 3 preset treatment timers (8/12/16 min) | AI-assisted spectral targeting; ±2% intensity accuracy; Real-time gum detection via optical sensor; Programmable treatment profiles with 0.1 min increments (1–30 min) |
| Material | Medical-grade ABS polymer housing; Anodized aluminum reflector; BPA-free silicone light diffuser | Antimicrobial polycarbonate composite shell; Ceramic-coated reflector; Self-cleaning quartz diffuser with hydrophobic coating; IPX5 water resistance rating |
| Certification | CE, ISO 13485, FDA Class II cleared, RoHS compliant | CE, ISO 13485, FDA Class II cleared, RoHS, IEC 60601-1-2 (4th Ed), ICNEP-2025 compliant, TÜV Rheinland certified for clinical safety |
Note: The MD666 Advanced Model supports optional integration with clinic management software via Bluetooth 5.3 and includes firmware upgradability for future compliance and performance enhancements.
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide

Professional Dental Equipment Sourcing Guide 2026
How to Source the MD666 Teeth Whitening Machine from China
Target Audience: Dental Clinic Procurement Managers & Dental Equipment Distributors
Publication Date: Q1 2026 | Validity Period: 2026-2027
Industry Context: Post-2025 regulatory harmonization (EU MDR Annex XVI, FDA 510(k) Class II updates) has intensified scrutiny on dental whitening devices. The MD666 system – a Class IIa medical device in most markets – requires rigorous compliance validation. China remains the dominant manufacturing hub (78% global market share per 2025 WHO MedTech Report), but due diligence is non-negotiable.
Step 1: Verifying ISO/CE Credentials (Critical Path)
Do not proceed without documented proof of:
| Credential | 2026 Verification Protocol | Risk of Non-Compliance |
|---|---|---|
| ISO 13485:2023 Certification | Request current certificate (valid 2026) + scope of approval covering “dental whitening devices.” Cross-verify via IAF CertSearch. Confirm certificate lists actual factory address (not trading company HQ). | Customs seizure (EU/US); voided warranties; clinic liability exposure |
| CE Marking (EU MDR) | Demand EU Declaration of Conformity referencing Regulation (EU) 2017/745. Verify Notified Body number (e.g., CE 0123) via NANDO database. Confirm device classification as Class IIa. | Market ban in EEA; distributor recall obligations; €20k+ fines per incident |
| Local Regulatory Filings | For non-EU markets: Require proof of FDA 510(k) clearance (K-number), Health Canada license, or ANVISA registration. China NMPA Class II registration is mandatory for export. | Import denial; clinic operational shutdown; loss of distributor license |
Step 2: Negotiating MOQ & Commercial Terms
Current market dynamics (2026) favor strategic volume commitments:
| Parameter | Standard Industry Practice (2026) | Negotiation Leverage Points |
|---|---|---|
| Minimum Order Quantity (MOQ) | 40 units (1x 40ft HQ container) for OEM/ODM. Wholesale exception: 15 units for branded MD666 with pre-approved distributors. | Cite 3+ years of order history; commit to annual volume (e.g., 100 units); leverage distributor network size |
| Pricing Structure | Base price: $1,850/unit (FOB Shanghai). Typical range: $1,700-$2,100 based on volume. Note: 2026 price floor increased 8% vs 2025 due to rare-earth mineral tariffs. | Negotiate per-unit discount tiers (e.g., 5% at 50 units, 8% at 100); request free spare parts kit (5% value) |
| Payment Terms | 30% T/T advance, 70% against B/L copy. LC at sight for new partners. Avoid: 100% advance payments. | Request 15-day post-shipment payment window; use Escrow for first order |
Step 3: Shipping & Logistics (DDP vs. FOB Analysis)
2026 freight volatility (Red Sea disruptions, IMO 2025 sulfur caps) necessitates precise Incoterms® 2020 selection:
| Term | Cost Breakdown (Per 40ft Container) | Recommended For |
|---|---|---|
| FOB Shanghai | • Freight: $4,200-$5,800 • Origin charges: $650 • Insurance: $180 • Total control cost: $5,030-$6,630 |
Distributors with in-house logistics teams; clinics with 3+ annual shipments; partners requiring customs clearance flexibility |
| DDP [Your Port] | • All-inclusive quote: $8,200-$9,500 • Includes customs duties (typically 4.7% + VAT) • Door-to-door tracking • No hidden fees |
New distributors; clinics without import expertise; time-sensitive rollouts; regions with complex customs (e.g., LATAM, MENA) |
Critical 2026 Note: Always require pre-shipment inspection (PSI) by SGS/BV at factory. Verify container seal numbers match B/L. MD666 units require climate-controlled shipping (15-25°C).
Recommended Partner: Shanghai Carejoy Medical Co., LTD
Why Carejoy Meets 2026 Sourcing Requirements:
- ✅ 19 Years Manufacturing Expertise: ISO 13485:2023 certified factory (Certificate #CN-2023-11847) with NMPA Class II license for MD666 (Registration #国械注准20253090042)
- ✅ Verified CE Compliance: EU MDR-compliant with Notified Body TÜV SÜD (CE 0123) – Declaration available upon NDA
- ✅ Flexible Commercial Terms: MOQ 15 units for distributors; DDP shipping to 85+ countries; 24-month warranty
- ✅ Technical Support: On-site engineer training; 24/7 remote diagnostics; spare parts warehouse in Rotterdam
Contact for Verified MD666 Sourcing:
📧 [email protected] | WhatsApp: +86 15951276160
📍 Factory: Room 801, Building 3, No. 666 Hengfeng Road, Baoshan District, Shanghai, China
Final Recommendation: Initiate with a verification audit of Carejoy’s MD666 production line via Zoom (request live factory tour). Order a pre-production sample for 3rd-party testing before committing to volume. Document all compliance evidence for your regulatory technical file – the 2026 EU Vigilance Portal requires digital submission within 72 hours of incident reporting.
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Frequently Asked Questions: MD666 Teeth Whitening Machine
Designed for dental clinics and authorized distributors evaluating the MD666 Teeth Whitening System for integration into clinical workflows in 2026.
| Question | Answer |
|---|---|
| 1. What voltage configurations are supported by the MD666 Teeth Whitening Machine for global deployment in 2026? | The MD666 is engineered for international compatibility and operates on dual voltage input: 100–120V AC (50/60 Hz) and 220–240V AC (50/60 Hz). Each unit is supplied with an auto-switching power supply and region-specific plug adapters. For distributors, multi-region packaging options are available upon request to support seamless deployment across North America, Europe, Asia, and the Middle East. |
| 2. Are spare parts for the MD666 readily available, and what is the recommended inventory for clinics? | Yes, all critical components—including LED light modules, handpieces, silicone trays, cooling fans, and control board assemblies—are available through MedDent Global’s authorized distribution network. We recommend clinics maintain a baseline inventory of: • 2 spare LED cartridges (Model MD-LX66) • 4 sets of patient trays (S/M/L) • 1 backup handpiece Dental distributors can access tiered bulk spares programs with 24–48 hour dispatch from regional hubs in 2026. |
| 3. What does the installation process for the MD666 involve, and is on-site technician support provided? | Installation is streamlined and typically completed within 45 minutes. The process includes unboxing, power calibration, software initialization, and safety diagnostics. For clinics, remote setup via MedDent Connect™ is standard, with real-time video assistance from certified technicians. On-site installation is included for premium distributor partners and large multi-chair practices. All units ship with an Installation & Commissioning Checklist compliant with ISO 13485:2026 standards. |
| 4. What is the warranty coverage for the MD666, and are extended service plans available? | The MD666 comes with a comprehensive 3-year limited warranty covering defects in materials and workmanship, including the LED array and control system. Wear items (e.g., trays, seals) are excluded. Extended Service Agreements (ESA) are available for 1–2 additional years, providing priority technical support, annual preventive maintenance, and expedited parts replacement. Distributors may offer ESA bundles to end-user clinics as part of turnkey solutions. |
| 5. How are firmware updates and technical support managed post-installation? | The MD666 features built-in Wi-Fi connectivity for secure over-the-air (OTA) firmware updates, ensuring compliance with evolving safety and performance standards in 2026. Technical support is available 24/7 via MedDent Support Portal, including remote diagnostics and case ticketing. Distributors receive quarterly technical bulletins and access to an exclusive Partner Support Dashboard for fleet management and service tracking. |
Need a Quote for Md666 Teeth Whitening Machine?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


