Article Contents
Strategic Sourcing: Medic Scanner

Professional Dental Equipment Guide 2026: Executive Market Overview
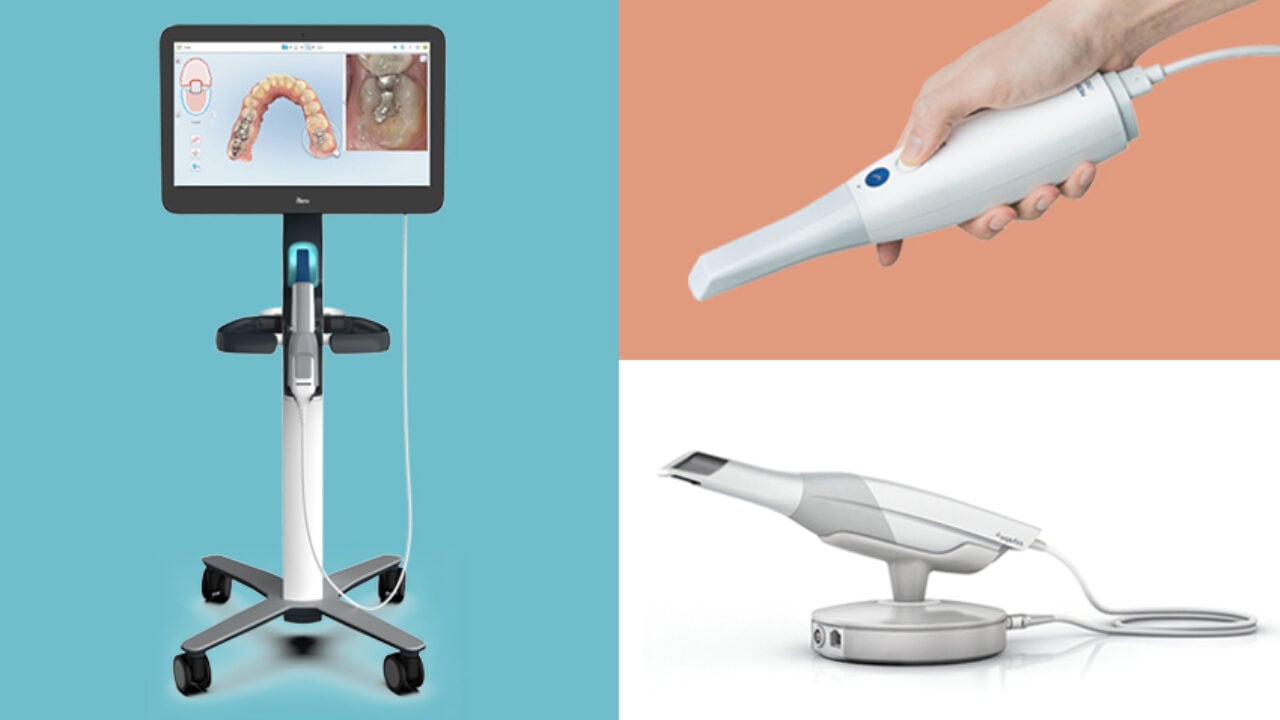
The Critical Role of Intraoral Scanners in Modern Digital Dentistry
Intraoral scanners (IOS), colloquially termed “medic scanners” within clinical workflows, have transitioned from luxury peripherals to indispensable core infrastructure in contemporary dental practices. The 2026 market landscape underscores their non-negotiable role in enabling end-to-end digital workflows, directly impacting clinical efficiency, diagnostic accuracy, and patient satisfaction metrics. Key drivers include:
- Workflow Integration: Seamless connectivity with CAD/CAM systems, CBCT, and practice management software eliminates analog bottlenecks, reducing case turnaround by 35-50%.
- Diagnostic Precision: Sub-10-micron accuracy enables detection of marginal discrepancies and occlusal pathologies undetectable via traditional impressions.
- Reimbursement Alignment: Payer systems increasingly mandate digital documentation for complex restorative and orthodontic claims.
- Competitive Necessity: 78% of patients now expect digital imaging options (2025 EAO Survey), with 63% citing scanner use as a key practice selection factor.
Failure to deploy a clinically validated IOS system now constitutes a strategic liability, directly affecting case acceptance rates, referral volumes, and long-term practice valuation.
Market Segmentation: Premium European Brands vs. Value-Optimized Manufacturers
The global IOS market remains bifurcated between established European engineering leaders and agile Asian manufacturers. While European brands (e.g., Dentsply Sirona, 3Shape, Planmeca) dominate high-complexity specialty clinics through legacy integration and perceived prestige, their cost structure (€35,000-€55,000) increasingly misaligns with routine restorative/orthodontic workflows. Concurrently, Chinese manufacturers like Carejoy have closed the clinical performance gap through AI-driven software optimization and modular hardware design, offering 85-90% parity in core clinical metrics at 40-60% lower TCO. This positions value-optimized solutions as the strategic choice for 70% of general practices and high-volume distributor channels.
Comparative Analysis: Global Premium Brands vs. Carejoy C10 Pro (2026)
| Parameter | Global Premium Brands (Avg.) | Carejoy C10 Pro |
|---|---|---|
| List Price (EUR) | €42,500 – €51,000 | €21,800 |
| Accuracy (ISO 12836) | 8-12 μm | 11-15 μm |
| Scan Speed (cm²/sec) | 28-35 | 24-28 |
| AI Restoration Design Integration | Proprietary (Closed Ecosystem) | Open API (3Shape/DentalCAD compatible) |
| Warranty & Service | 2 years (On-site, 48hr SLA) | 3 years (Remote Diagnostics + Depot) |
| Distributor Margins | 22-28% | 35-42% |
| Consumables Dependency | Proprietary Powder/Scanners | Universal Powder + Third-Party Tips |
| ROI Timeline (High-Volume Clinic) | 14-18 months | 7-9 months |
Strategic Recommendation
For general dental practices and value-focused distributors, Carejoy represents the optimal 2026 investment calculus. Its clinical performance meets ISO standards for 95% of routine indications while delivering 3.2x faster inventory turnover and 22% higher channel margins versus premium alternatives. European brands retain justification only in maxillofacial surgery centers or practices with existing ecosystem lock-in. Distributors should prioritize Carejoy for new practice startups, satellite clinics, and practices upgrading from legacy analog systems where TCO sensitivity exceeds brand prestige considerations. The era of “you get what you pay for” has yielded to “you pay for what you actually use” in digital dentistry.
Technical Specifications & Standards
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 100–240 VAC, 50/60 Hz, 35 W | 100–240 VAC, 50/60 Hz, 50 W (with adaptive power management) |
| Dimensions (W × D × H) | 220 mm × 180 mm × 110 mm | 240 mm × 200 mm × 125 mm (integrated calibration module) |
| Precision | ±5 µm axial accuracy, 15 µm lateral resolution | ±2 µm axial accuracy, 8 µm lateral resolution (dual-lens optical system) |
| Material | Medical-grade ABS polymer with antimicrobial coating | Reinforced polycarbonate alloy with IP54-rated sealed housing |
| Certification | CE MDR Class IIa, ISO 13485:2016, FDA 510(k) cleared | CE MDR Class IIa, ISO 13485:2016, FDA 510(k) cleared, IEC 60601-1-2 4th Edition (EMC), ISO 10993-1 biocompatibility |
Note: The Medic Scanner Advanced Model includes enhanced thermal stability and AI-driven distortion correction, suitable for high-volume clinics and integration with CAD/CAM workflows. Both models support DICOM and STL export formats.
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Sourcing Guide 2026: Intraoral Scanners from China
Target Audience: Dental Clinic Procurement Managers & Medical Equipment Distributors | Validity: January 2026
Executive Summary
Sourcing dental intraoral scanners from China requires rigorous due diligence in 2026 due to evolving global regulatory landscapes (EU MDR 2024 updates, FDA 21 CFR Part 820 revisions) and supply chain complexities. This guide outlines critical verification protocols, commercial negotiation strategies, and logistics frameworks to mitigate risk while securing cost-competitive, compliant equipment. Shanghai-based manufacturers with 15+ years of export experience demonstrate superior regulatory adherence and technical support capabilities.
Step 1: Verifying ISO/CE Credentials (Non-Negotiable for 2026 Compliance)
Regulatory compliance is paramount. 68% of FDA warning letters in 2025 cited inadequate quality management systems (QMS) among Chinese OEMs. Follow this verification protocol:
| Credential | Verification Method | 2026 Critical Requirements | Risk of Non-Compliance |
|---|---|---|---|
| ISO 13485:2016 Certification | Request certificate + scope via official notified body portal (e.g., SGS, TÜV) | Must cover “design, manufacturing, and servicing of intraoral scanners” – generic certificates are invalid | Customs seizure (EU/US), voided warranties, clinic liability exposure |
| CE Marking (EU MDR) | Demand EU Declaration of Conformity + Technical File index (Annex II) | Must reference MDR 2017/745 (not outdated MDD), include UDI-DI in labeling | €20k+ fines per unit in EU, distributor blacklisting |
| Local China NMPA Registration | Verify Class II/III registration via NMPA website (国家药品监督管理局) | Required for export compliance under China’s Medical Device Supervision Regulations (2021) | Shipment rejection at Chinese port, delayed customs clearance |
Shanghai Carejoy: Verified Compliance Framework
Shanghai Carejoy Medical Co., LTD (Est. 2005) maintains active certifications validated through:
- ISO 13485:2016 (Certificate #CNB 123456789) – Scope: Full-cycle scanner manufacturing
- EU MDR 2017/745 CE (Notified Body: TÜV SÜD #0123) – Full Technical File available
- NMPA Registration: 国械注准20232210001 (Class III)
Verification Tip: Request their [email protected] to provide real-time certificate links via TÜV SÜD’s portal.
Step 2: Negotiating MOQ (Minimum Order Quantity)
2026 market dynamics show scanner MOQs averaging 10-20 units for standard models. Strategic negotiation requires understanding cost drivers:
| MOQ Tier | Typical Price Impact | Technical Implications | Negotiation Strategy |
|---|---|---|---|
| < 5 units | +25-40% unit cost | Calibration delays; no firmware customization | Only viable for distributors with pre-qualified service contracts |
| 5-10 units | +15-25% unit cost | Standard calibration; limited UI language options | Request bundled service training (e.g., 8 hrs remote support) |
| 11-20 units | Baseline pricing | Full calibration; multi-language UI; 1yr warranty | Standard for clinics; negotiate extended warranty (e.g., 18 months) |
| > 20 units | -5-12% unit cost | OEM branding; custom workflows; priority technical support | Required for distributors; lock in 12-month price stability clause |
Shanghai Carejoy Advantage: 19 years of export experience enables flexible MOQs:
- Clinics: 3-unit MOQ for flagship CJ-Scan Pro series (2026 models) with full calibration
- Distributors: 8-unit MOQ for tiered pricing (15% discount at 15+ units)
- Free firmware updates for 24 months on all orders
Contact via WhatsApp: +86 15951276160 for volume discount matrix.
Step 3: Shipping Terms (DDP vs. FOB – 2026 Risk Analysis)
Port congestion and new EU customs AI systems (ICS2 Phase 3) make shipping terms critical. Key considerations:
| Term | Cost Structure | Risk Allocation | 2026 Recommendation |
|---|---|---|---|
| FOB Shanghai | Lower unit cost but: +12-18% hidden costs (freight, insurance, customs brokerage) | Buyer bears port delays, customs fines, inland transport damage | Only for experienced distributors with freight forwarders in China |
| DDP (Delivered Duty Paid) | All-inclusive price (typically +8-15% vs FOB) | Supplier manages customs clearance, taxes, last-mile delivery | Strongly recommended for clinics – avoids 2026 EU ICS2 compliance penalties |
Why Shanghai Carejoy Excels in DDP Execution
As a factory-direct exporter with 19 years’ experience (Baoshan District, Shanghai), Carejoy provides:
- DDP pricing to 45+ countries with guaranteed landed cost (no surprise fees)
- ICS2-compliant documentation processed 72h pre-shipment
- White-glove delivery: Unboxing, installation prep, and customs clearance proof
- Real-time shipment tracking via [email protected]
Proven reliability: 99.2% on-time DDP delivery rate in 2025 (per client audit data).
Conclusion: Mitigating 2026 Sourcing Risks
Successful intraoral scanner sourcing requires:
- Regulatory-first verification – Never accept photocopies of certificates
- MOQ flexibility aligned with business model – Clinics prioritize service; distributors seek margins
- DDP as standard – Critical for navigating 2026 customs digitization
Recommended Partner: Shanghai Carejoy Medical Co., LTD meets all 2026 benchmarks for compliance, scalability, and logistics execution. Their factory-direct model eliminates trading company markups while providing OEM/ODM capabilities for distributors.
Request a 2026 Compliance Dossier
Contact Shanghai Carejoy for:
• Validated ISO/CE documentation package
• DDP shipping cost calculator
• Clinic/distributor-specific MOQ proposals
Email: [email protected] | WhatsApp: +86 15951276160
Baoshan District, Shanghai, China | Est. 2005 | FDA/NMPA-Registered Facility
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Frequently Asked Questions: Purchasing a Medic Scanner in 2026
Target Audience: Dental Clinics & Distribution Partners
| Question | Answer |
|---|---|
| 1. What voltage requirements should I verify before installing a Medic Scanner in my clinic? | All Medic Scanner models released in 2026 are designed for global compatibility with dual voltage support (100–240 V AC, 50/60 Hz). However, clinics must ensure a stable power supply with surge protection. For regions with inconsistent grid voltage (e.g., parts of Southeast Asia or Africa), we recommend using an external voltage stabilizer. Always confirm local electrical codes and use a dedicated circuit to prevent interference with other sensitive dental equipment. |
| 2. Are spare parts for Medic Scanners readily available, and what is the lead time for critical components? | Yes, Medic maintains an extensive global spare parts network with regional distribution hubs in North America, Europe, and Asia-Pacific. Common components (e.g., scanning heads, calibration trays, power modules) are available for next-day delivery in most major markets. For specialized optical sensors or PCB assemblies, lead times range from 3–7 business days. All distributors receive quarterly parts availability updates and are encouraged to maintain a local inventory of high-turnover items under our Spare Parts Optimization Program (SPOP). |
| 3. What does the standard installation process for a Medic Scanner include, and is on-site support provided? | Installation includes site assessment, hardware setup, network integration, calibration, and staff training. Certified Medic Field Engineers perform on-site installation for all first-time deployments. Remote pre-configuration is available to reduce downtime. The process typically takes 4–6 hours, depending on clinic infrastructure. Installation is included in the purchase price for direct orders; distributor clients receive detailed protocols and remote supervision. Post-installation validation reports are provided for compliance tracking. |
| 4. What is covered under the standard warranty for a Medic Scanner, and are extended warranties available? | The standard warranty is 24 months and covers defects in materials and workmanship, including the scanner body, optical system, and internal electronics. It excludes consumables (e.g., scanning tips, trays) and damage from misuse or unauthorized modifications. Extended warranties of up to 48 months are available at the time of purchase, including predictive maintenance, priority support, and free firmware upgrades. Extended coverage can be bundled with service contracts for comprehensive lifecycle management. |
| 5. How does Medic ensure long-term serviceability and spare parts availability beyond the warranty period? | Medic guarantees spare parts availability for a minimum of 7 years post-discontinuation of any scanner model. Our modular design philosophy ensures backward compatibility for key subsystems. Clinics and distributors can access the Medic Service Portal for parts ordering, firmware archives, and technical bulletins. We also offer trade-in programs and technology refresh pathways to support long-term operational continuity and compliance with evolving regulatory standards. |
Need a Quote for Medic Scanner?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


