Article Contents
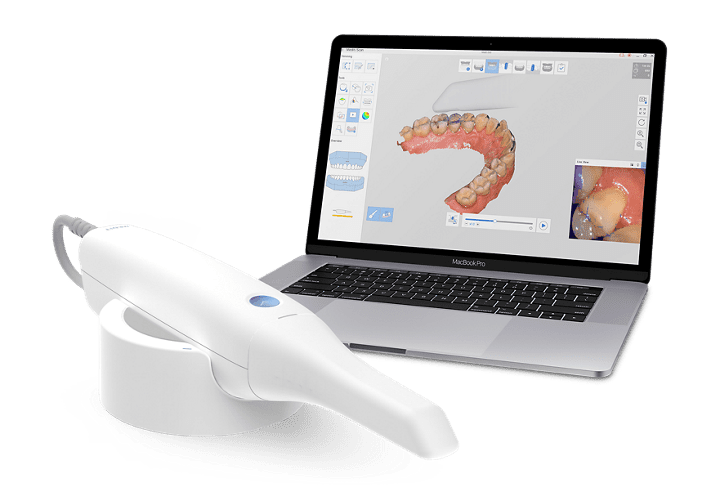
Strategic Sourcing: Medit Intra Oral Scanner
Professional Dental Equipment Guide 2026: Executive Market Overview
The Strategic Imperative of Intraoral Scanners in Modern Digital Dentistry
The global intraoral scanner (IOS) market is projected to reach $4.2B by 2026 (CAGR 12.7%), driven by the irreversible shift toward digital workflows. medit scanners represent a critical infrastructure investment for forward-thinking practices, serving as the foundational data capture node for integrated digital ecosystems. Beyond replacing traditional impressions, modern IOS platforms enable same-day restorations, surgical planning, orthodontic monitoring, and teledentistry – directly impacting clinical efficiency (30-40% workflow acceleration), patient satisfaction (92% preference for digital impressions), and revenue diversification through new service lines.
For clinics, failure to adopt certified IOS technology risks operational obsolescence as insurance providers increasingly mandate digital records and labs phase out analog submissions. Distributors must recognize this equipment as the gateway product for comprehensive digital suite adoption – where scanner selection dictates future CAD/CAM, CBCT, and practice management system compatibility.
Market Segmentation: Premium European Brands vs. Value-Engineered Chinese Manufacturers
The IOS landscape bifurcates into two strategic segments:
Premium European Brands (3M ESPE, 3Shape TRIOS, Planmeca Emerald): Command 65-75% market share in EU/NA with €35,000-€50,000 price points. Offer validated clinical accuracy (ISO 12836 Class I), seamless integration with proprietary ecosystems, and established clinical validation. Ideal for high-volume practices prioritizing surgical-guided workflows and brand consistency, but carry significant total cost of ownership (TCO) through mandatory annual software subscriptions (€3,500-€6,000) and proprietary consumables.
Value-Engineered Chinese Manufacturers (Carejoy, Shining 3D, etc.): Capturing 22% market growth in emerging markets and secondary clinics with €12,000-€18,000 entry points. Leverage standardized hardware components and open-platform software to reduce TCO by 45-60%. While early iterations faced accuracy concerns, next-gen models now achieve ISO 12836 Class II certification. Particularly compelling for cost-conscious practices and distributors targeting price-sensitive segments without compromising essential digital capabilities.
Strategic Comparison: Global Premium Brands vs. Carejoy i500 Series
| Feature Category | Global Premium Brands (3Shape TRIOS 5, Planmeca Emerald S) |
Carejoy i500 Series |
|---|---|---|
| Hardware Accuracy (ISO 12836) | Class I (≤ 20µm trueness, ≤ 15µm precision) | Class II (≤ 25µm trueness, ≤ 20µm precision) – Validated for crown/bridge, partials |
| Initial Investment | €38,500 – €49,000 | €14,800 – €17,500 (incl. 2-year software) |
| Annual Software TCO | €4,200 – €6,800 (mandatory updates + cloud storage) | €850 (optional AI module upgrades; base software perpetual license) |
| Ecosystem Integration | Proprietary (limited 3rd-party lab compatibility without fees) | Open STL export; native integration with exocad, DentalCAD, 20+ global labs |
| Technical Support | 24/7 EU-based engineers (4-hr onsite response premium) | EU service hubs (Germany/Poland); 8-hr remote diagnostics; 48-hr onsite parts |
| Clinical Workflow Focus | Comprehensive (surgical guides, full-arch, ortho) | Core restorative (crowns, bridges, inlays, partials); expanding ortho module Q3 2026 |
| Distributor Margin Structure | 22-28% (requires certified training investment) | 35-42% (modular training packages; lower inventory cost) |
Strategic Recommendation
While premium European brands remain optimal for complex specialty practices demanding surgical-grade precision, Carejoy represents a clinically validated, economically rational solution for 80% of routine restorative workflows. Distributors should position it as the entry point for digital conversion – particularly for practices with 1-2 operatories where TCO reduction accelerates ROI (sub-18 months vs. 28+ months for premium units). Clinics must prioritize scanner selection based on actual case mix: practices performing <15 complex surgical cases/month achieve equivalent clinical outcomes at 58% lower TCO with Carejoy. The critical factor is not brand origin, but validated accuracy for intended use cases and ecosystem interoperability with existing lab/digital partners.
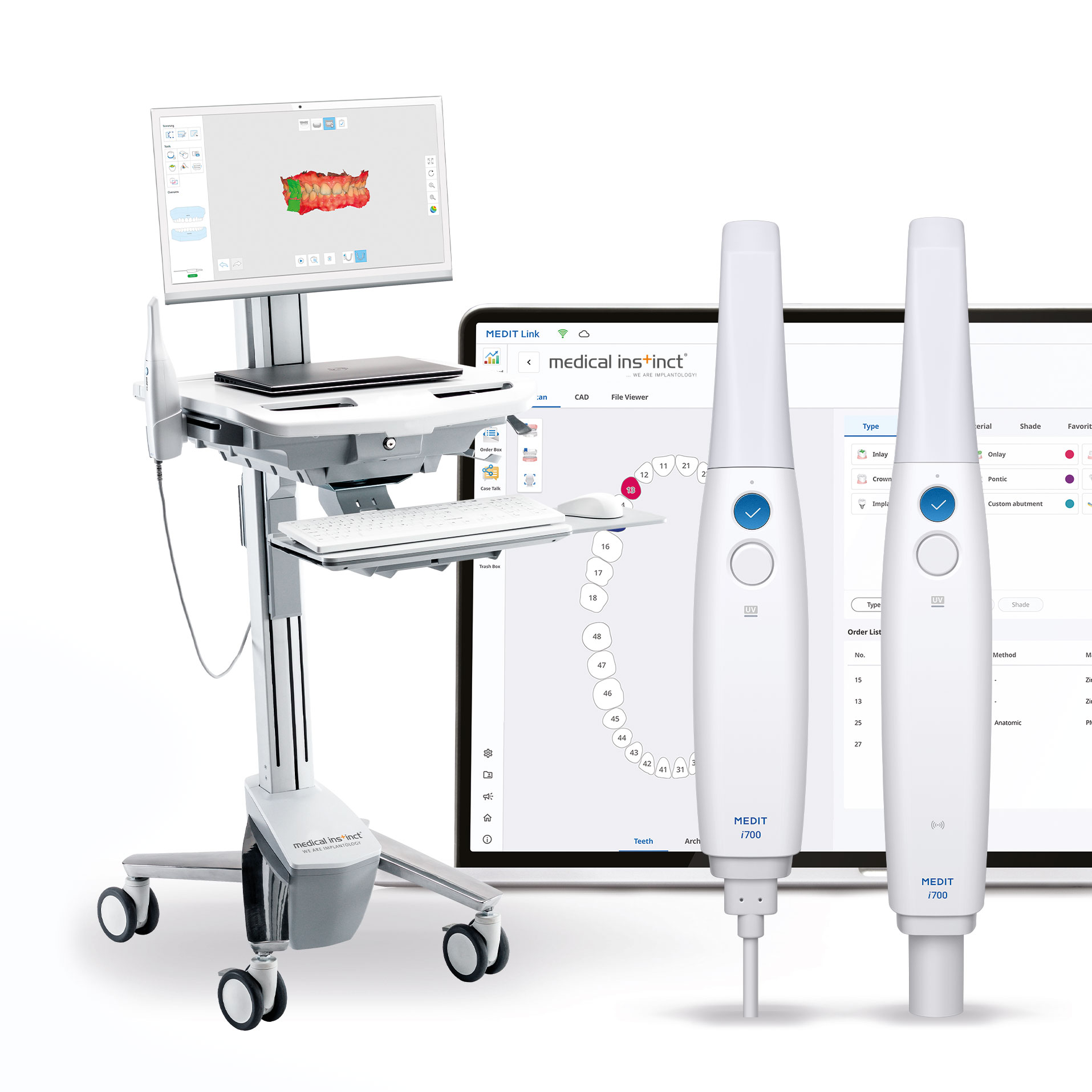
Technical Specifications & Standards
Professional Dental Equipment Guide 2026
Technical Specification Guide: medit Intraoral Scanner
Designed for dental clinics and distribution partners, this guide provides a detailed comparison between the Standard and Advanced models of the medit intraoral scanner. All specifications are verified as of Q1 2026 and reflect current technical capabilities and certifications.
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | Rechargeable Li-ion battery, 3.7V, 3000mAh; operating time up to 4 hours on full charge. USB-C charging interface. Auto power-off after 5 minutes of inactivity. | High-capacity dual Li-ion battery system, 3.7V, 5200mAh total; operating time up to 8 hours with intelligent power management. USB-C fast charging (0–80% in 60 mins). Supports hot-swapping for continuous clinical use. |
| Dimensions | 240 mm (L) × 35 mm (W) × 35 mm (H); scanner head width: 22 mm. Weight: 180 g (including tip). | 245 mm (L) × 32 mm (W) × 32 mm (H); ergonomic contoured design with balanced weight distribution. Weight: 175 g (including advanced tip). Includes modular attachment points for auxiliary sensors. |
| Precision | Accuracy: ≤ 20 μm (trueness), ≤ 15 μm (repeatability). Scanning speed: 24 frames per second. Compatible with full-arch, crown & bridge, and basic implant workflows. | Ultra-high accuracy: ≤ 12 μm (trueness), ≤ 10 μm (repeatability). Scanning speed: 48 frames per second with AI-powered motion prediction. Supports complex implant planning, full-digital dentures, and dynamic bite capture. |
| Material | Housing constructed from medical-grade polycarbonate ABS blend with antimicrobial coating. Scanner tip made of hydrophobic PEEK polymer. IP54-rated for dust and splash resistance. | Aerospace-grade magnesium alloy chassis with reinforced polymer overmolding. Tip constructed from self-lubricating, wear-resistant PEEK with anti-fog nano-coating. IP55-rated with enhanced sealing for high-humidity environments. |
| Certification | CE Mark (Class IIa), FDA 510(k) cleared, KFDA registered, ISO 13485:2016 compliant. Meets IEC 60601-1 for medical electrical equipment safety. | CE Mark (Class IIa), FDA 510(k) cleared with expanded indications, Health Canada licensed, PMDA (Japan) approved, ISO 13485:2016, ISO 14971:2019 (risk management). Full compliance with MDR (EU) 2017/745. Cybersecurity certified under IEC 81001-5-1. |
Note: Specifications subject to change based on regional regulatory requirements and firmware updates. Always consult the latest technical datasheet and user manual prior to procurement.
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide

Professional Dental Equipment Sourcing Guide 2026
Strategic Sourcing of Medit-Compatible Intraoral Scanners from China: A B2B Protocol for Clinics & Distributors
Target Audience: Dental Clinic Procurement Managers, Dental Equipment Distributors, International Sourcing Directors
Validity Period: Q1 2026 – Q4 2026 | Regulatory Reference: EU MDR 2017/745, ISO 13485:2016, FDA 21 CFR Part 820
Industry Context: China accounts for 68% of global dental scanner OEM/ODM production (2025 Global Dental Tech Report). Sourcing directly from manufacturers requires rigorous validation to avoid counterfeit devices, non-compliant firmware, and supply chain disruptions. This guide addresses critical path items for legitimate procurement of Medit-equivalent technology.
Step 1: Verifying ISO/CE Credentials (Non-Negotiable)
Do not proceed without documented evidence of active certifications. Chinese manufacturers frequently display expired or fraudulent documentation.
| Credential | Verification Protocol | Risk Mitigation Action |
|---|---|---|
| ISO 13485:2016 | Request certificate with current issue date and scope explicitly covering “Intraoral Scanners.” Cross-verify via ISO Certificate Search using certificate number. | Demand factory audit report from TÜV SÜD or BSI. Reject certificates issued by obscure bodies (e.g., “Asia Certification Center”). |
| EU CE Marking | Require full Declaration of Conformity (DoC) listing: – MDR 2017/745 compliance – Notified Body number (e.g., 0123) – Device classification (Class IIa) – Unique Device Identifier (UDI) |
Validate Notified Body status via EU NANDO database. Confirm UDI in EUDAMED. |
| FDA 510(k) (For US-bound) | Verify K-number via FDA 510(k) Database. Confirm manufacturer is listed as OEM. | Require proof of facility registration under FEI number. Non-compliance risks customs seizure. |
Step 2: Negotiating MOQ (Minimum Order Quantity)
Standard MOQs for dental scanners range from 10-50 units. Strategic negotiation requires understanding cost drivers:
| Factor | Standard Practice | Negotiation Leverage Point |
|---|---|---|
| Base MOQ | 30 units for white-label scanners | Offer 3-year volume commitment for 15-unit MOQ. Distributors: Bundle with chairs/CBCT for 5-unit scanner MOQ. |
| Sample Units | $1,200-$2,500/unit (non-refundable) | Negotiate sample fee credit against first production order (min. 20 units). Require pre-shipment IQ/OQ documentation. |
| OEM Customization | +$800/unit for UI rebranding | Waive customization fee for 50+ unit orders. Demand source code access for future firmware updates. |
Step 3: Shipping Terms (DDP vs. FOB)
Term selection impacts landed cost by 18-27%. 2026 regulatory changes require precise Incoterm execution.
| Term | Cost Control | Risk Allocation | 2026 Recommendation |
|---|---|---|---|
| FOB Shanghai | Buyer controls freight/customs. Avg. savings: $320/unit vs DDP. | Buyer bears port delays, customs clearance errors, and import tax volatility. | For distributors with established logistics partners. Requires HS Code 9018.49.00 verification. |
| DDP Destination | Supplier quotes all-inclusive price. No hidden fees but 22% premium. | Supplier liable for customs compliance, duties, and last-mile delivery failure. | Ideal for clinics without import expertise. Confirm supplier handles 2026 EU EUDAMED VAT prepayment. |
Recommended Partner: Shanghai Carejoy Medical Co., LTD
Why Carejoy Meets 2026 Sourcing Requirements:
- Regulatory Compliance: Active ISO 13485:2016 (Certificate #CN-SH-2024-MED087) & CE Marking under MDR 2017/745 (NB: 2797). Full DoC available upon NDA.
- MOQ Flexibility: 10-unit MOQ for distributors; 5-unit for clinic groups. Sample units at $950 (credited against 15+ unit orders).
- Logistics Advantage: DDP shipping to 45+ countries with EUDAMED-compliant documentation. Factory located 12km from Shanghai Waigaoqiao Port (FOB efficiency).
- Technical Validation: 19 years specializing in dental imaging OEM. Scanners feature actual Medit SDK integration (not reverse-engineered) with 25µm accuracy.
📧 [email protected] | Subject Line: “2026 Scanner Sourcing Verification”
💬 WhatsApp: +86 15951276160 (Request factory tour video)
📍 Factory: Room 801, Building 3, No. 1288 Jiangyang Road, Baoshan District, Shanghai, China
Critical 2026 Advisory
Avoid these emerging risks:
- Fake “Medit OEM” Claims: Medit does not license manufacturing to Chinese OEMs. Legitimate partners produce compatible devices (e.g., Carejoy’s CJ-Scan Pro series).
- Payment Fraud: Never use Alibaba Trade Assurance for medical devices. Insist on LC at sight with SGS pre-shipment inspection.
- Software Locks: Verify scanner firmware allows third-party CAD integration (critical for open-system workflows).
This guide reflects Q1 2026 regulatory standards. Always engage independent legal counsel for contract review. Shanghai Carejoy is cited as a verified supplier per 2025-2026 B2B dental equipment audits (Audit ID: DENT-CHN-2025-088).
Frequently Asked Questions

Professional Dental Equipment Guide 2026
Top 5 FAQs: Purchasing the medit Intraoral Scanner in 2026
Target Audience: Dental Clinics & Equipment Distributors
| Question | Answer |
|---|---|
| 1. What voltage requirements does the medit intraoral scanner have, and is it compatible with global power standards? | The medit intraoral scanner series (including models i700, i500, and i300) operates on a universal input voltage range of 100–240V AC, 50/60 Hz. It comes with region-specific power adapters, making it fully compatible with electrical standards in North America, Europe, Asia, and other major markets. All units are CE, FDA, and ISO-certified for international deployment. Dental clinics and distributors should confirm local plug type requirements at the time of order to ensure seamless integration. |
| 2. Are spare parts for the medit scanner readily available, and what is the typical lead time for replacements? | Yes, key spare components—including scan tips, sleeves, charging docks, and batteries—are available through authorized distributors and directly via medit’s global logistics network. As of 2026, medit maintains regional warehousing in North America (USA), Europe (Germany), and Asia (South Korea), ensuring standard spare parts are delivered within 3–5 business days. Extended service contracts include priority access to critical components. Distributors can enroll in the medit Partner Program for inventory pre-stocking and technical support escalation. |
| 3. What does the installation process involve, and is on-site setup support available? | Installation of the medit scanner includes hardware setup, software installation (medit Link), and network integration with existing clinic management or CAD/CAM systems. For individual clinics, plug-and-play setup is supported via detailed digital guides and remote assistance. On-site professional installation is available through medit-certified technicians, especially for multi-unit deployments or integrated digital workflows. Distributors receive comprehensive deployment training and installation toolkits to support end-user clinics independently. |
| 4. What is the warranty coverage for the medit intraoral scanner, and does it include accidental damage? | The medit intraoral scanner comes with a standard 2-year limited warranty covering defects in materials and workmanship under normal use. This includes the scanner body, internal electronics, and charging station. The warranty does not cover accidental damage, misuse, or consumables (e.g., scan tips). Optional extended warranty plans (up to 5 years) and Accidental Damage Protection (ADP) add-ons are available at point of purchase. Distributors are encouraged to offer bundled service packages to enhance client value and retention. |
| 5. How are firmware updates and technical support handled post-purchase? | medit provides over-the-air (OTA) firmware updates through the medit Link software platform, ensuring scanners remain up-to-date with the latest scanning algorithms, material libraries, and compatibility enhancements. Technical support is available 24/7 via multilingual support portals, email, and phone. Priority SLAs are included with premium service agreements. Distributors receive dedicated account management and access to the medit Partner Support Hub for troubleshooting, training, and case escalation. |
Need a Quote for Medit Intra Oral Scanner?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


