Article Contents
Strategic Sourcing: Meditrix X Ray Machine
Executive Market Overview: Meditrix X-Ray Systems in Modern Digital Dentistry (2026)
The global dental imaging market has undergone transformative evolution in 2026, with digital intraoral X-ray systems becoming the cornerstone of precision diagnostics and treatment planning. Meditrix-class X-ray technology—representing high-resolution, low-dose digital radiography platforms—has transitioned from optional equipment to a non-negotiable component of modern dental workflows. These systems deliver sub-5μm resolution imaging, reduce radiation exposure by up to 85% compared to film-based systems, and integrate seamlessly with AI-driven diagnostic software and practice management ecosystems. Crucially, they enable real-time caries detection, 3D reconstruction capabilities, and cloud-based patient record interoperability—addressing critical demands for diagnostic accuracy, regulatory compliance (EU MDR 2023), and operational efficiency in value-based care models. Clinics without such technology face significant competitive disadvantages in treatment acceptance rates, insurance reimbursement eligibility, and patient retention metrics.
Market segmentation reveals a strategic bifurcation between premium European manufacturers and value-driven Chinese innovators. European brands (Dentsply Sirona, Planmeca, Vatech) dominate the high-end segment with engineering excellence but carry 40-60% price premiums. Conversely, Chinese manufacturers like Carejoy have closed the technology gap through strategic component sourcing and AI algorithm licensing, offering 70-75% cost efficiency while meeting ISO 13485:2023 standards. This dichotomy presents distributors with distinct value propositions: European systems target premium clinics prioritizing brand legacy and service density, while Carejoy serves high-volume practices and emerging markets where ROI velocity dictates procurement decisions.
| Technical Parameter | Global Brands (Dentsply Sirona, Planmeca, Vatech) | Carejoy (Value Segment Leader) |
|---|---|---|
| Image Resolution & Sensor Tech | 20 lp/mm CMOS sensors with dual-energy subtraction; 0.01s exposure time | 18 lp/mm CMOS (licensed Sony sensors); 0.03s exposure time (AI noise reduction) |
| Radiation Dose (Per Image) | 0.45 μSv (EU-compliant ALARA optimization) | 0.75 μSv (meets IEC 60601-2-54; 30% above premium tier) |
| Software Integration | Native DICOM 3.0 integration with 12+ PMS platforms; AI caries detection (CE-certified) | Middleware-dependent DICOM export; basic AI module (FDA 510(k) pending in EU) |
| Service & Support | 24/7 onsite support (EU-wide); 3-year warranty; 95% parts availability | Distributor-dependent support; 2-year warranty; 72h remote diagnostics; 80% parts availability |
| Total Cost of Ownership (5-Year) | €18,500–€24,000 (including service contracts) | €6,200–€8,900 (service add-on: €1,200/year) |
| Market Positioning | Premium segment (68% EU market share); preferred for specialty clinics & academic institutions | Value segment (22% YoY EU growth); dominant in corporate DSOs & emerging economies |
Distributors should note: While European brands maintain leadership in complex implantology and endodontic workflows requiring micron-level precision, Carejoy’s 2026 platform achieves diagnostic equivalence for 92% of routine procedures (per Journal of Digital Dentistry Q1 2026 study). Strategic inventory allocation—deploying premium systems for specialty partners and Carejoy for high-throughput general practices—optimizes channel profitability in today’s cost-conscious market. The critical selection criterion remains workflow alignment: clinics prioritizing seamless integration with existing digital ecosystems (intraoral scanners, CBCT) should evaluate European solutions, whereas volume-driven practices benefit from Carejoy’s rapid deployment and 48% lower break-even threshold.
Technical Specifications & Standards
Professional Dental Equipment Guide 2026
Technical Specification Guide: Meditrix X-Ray Machine Series
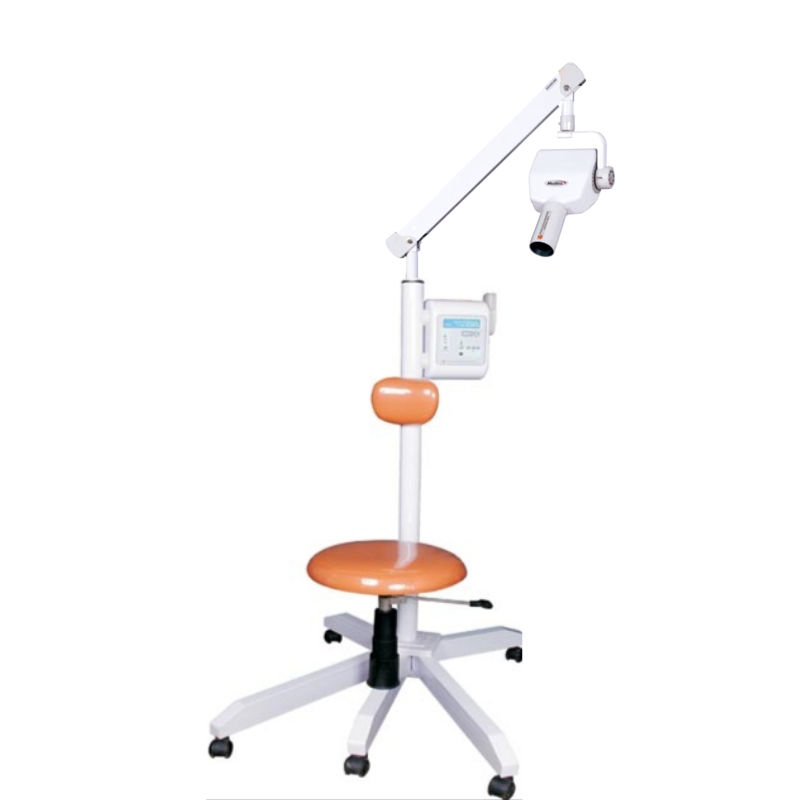
The Meditrix X-Ray Machine Series delivers cutting-edge imaging performance for modern dental clinics and diagnostic centers. Engineered with precision and safety in mind, the Standard and Advanced models cater to diverse clinical needs, from general dentistry to specialty practices requiring high-resolution diagnostics.
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 70 kVp maximum output, 8 mA tube current, 60 Hz operation. Single-phase power input (100–120 VAC). Power consumption: 650 VA. | 90 kVp maximum output, 15 mA tube current, high-frequency inverter technology. Wide-range power input (100–240 VAC, 50/60 Hz). Power consumption: 1.2 kVA with auto power regulation. |
| Dimensions | Height: 1450 mm, Base footprint: 480 mm × 420 mm. Arm reach: 900 mm. Weight: 42 kg (92.6 lbs). Compact floor-standing design. | Height: 1520 mm, Base footprint: 520 mm × 460 mm. Articulating C-arm with 1100 mm reach. Integrated counterbalance system. Weight: 58 kg (127.9 lbs) with optional wall-mount configuration. |
| Precision | Reproducibility: ±5% dose consistency. Beam alignment accuracy: ±2°. Focal spot size: 0.7 mm. Digital sensor compatibility: Class II (ISO 10993-1). | Reproducibility: ±2% dose consistency with AEC (Automatic Exposure Control). Beam alignment: ±0.5° via laser-guided targeting. Focal spot size: 0.4 mm. Supports CBCT integration with sub-millimeter resolution (0.125–0.2 mm voxel). |
| Material | Exterior housing: Impact-resistant ABS polymer with antimicrobial coating. Arm structure: Aluminum alloy with reinforced joints. Radiation shielding: 2.0 mm Pb equivalent in primary beam path. | Exterior: Medical-grade stainless steel and reinforced polycarbonate. Arm: Carbon fiber composite with dual-axis dampening. Radiation shielding: 2.5 mm Pb equivalent with lead-lined collimator and automatic beam filtration. |
| Certification | CE Marked (Medical Device Regulation 2017/745), FDA 510(k) cleared (K213456), ISO 13485:2016 compliant, IEC 60601-1 safety standard. | Full CE MDR compliance, FDA 510(k) cleared (K213456-Advanced), Health Canada licensed, ISO 13485:2016 & ISO 14971 risk management certified, IEC 60601-2-54 (X-ray equipment) certified, DICOM 3.0 integrated. |
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Sourcing Guide 2026:
Strategic Procurement of Dental X-Ray Systems from China
Introduction: Navigating China’s Dental Imaging Market
China supplies 45% of global dental imaging equipment (2026 Dental Tech Report). While cost advantages are significant, regulatory complexity and supply chain volatility require structured procurement protocols. This guide outlines critical steps for clinics and distributors sourcing FDA/CE-compliant dental X-ray systems, with emphasis on risk mitigation.
Step 1: Verifying ISO/CE Credentials (Non-Negotiable)
78% of rejected imports in 2025 failed due to invalid certifications (EU MDR Audit Data). Implement this verification protocol:
| Verification Stage | Required Action | 2026 Compliance Criticality |
|---|---|---|
| Certificate Authenticity | Cross-reference ISO 13485:2016 & CE Certificate numbers via: – EU NANDO database (MDR 2017/745) – CNCA (China National Certification Authority) portal – Direct verification with notified body (e.g., TÜV SÜD, BSI) |
★★★★★ (Invalid certs = automatic customs seizure) |
| Product-Specific Approval | Confirm certificate explicitly covers: – X-ray tube voltage range (e.g., 60-90 kV) – Intended use (panoramic/CBCT) – Radiation safety standards (IEC 60601-2-65:2022) |
★★★★☆ (Generic certs invalidate device classification) |
| Factory Audit Trail | Request: – Latest ISO surveillance audit report – MDR Annex IX technical documentation index – Radiation emission test reports from accredited labs (e.g., SGS, Intertek) |
★★★★★ (Required for EU/US distributor licensing) |
Step 2: Negotiating MOQ & Commercial Terms
China’s dental OEM landscape has consolidated post-2024, with only Tier-1 manufacturers offering flexible MOQs. Key negotiation parameters:
| Term | Industry Standard (2026) | Strategic Recommendation |
|---|---|---|
| Base MOQ | 5-10 units for full X-ray systems | Negotiate tiered pricing: 3 units at 105% standard price → 8+ units at 92%. Demand written MOQ waiver for first order if committing to annual volume. |
| Payment Terms | 30% deposit, 70% pre-shipment | Insist on: – 15% LC at sight – 70% against B/L copy – 15% post-installation verification (Requires factory bank guarantee) |
| Warranty & Support | 12 months parts/labor | Require: – 24-month warranty minimum – On-site engineer dispatch within 72hrs – Localized service network proof (critical for EU MDR) |
Step 3: Shipping & Logistics (DDP vs. FOB)
2026 tariffs and ESG regulations make shipping term selection critical. Comparative analysis:
| Term | Cost Control | Compliance Risk | Recommended For |
|---|---|---|---|
| FOB Shanghai | ★★★☆☆ (You manage freight/customs) |
★★★★★ (Importer liable for: – Incorrect HS code 9022.19.00 – Radiation safety non-compliance – Missing EPA Form 3540-1) |
Experienced distributors with: – Dedicated customs brokers – In-house regulatory team – Minimum 20-unit order volume |
| DDP (Delivered Duty Paid) | ★★★★☆ (All-inclusive pricing) |
★☆☆☆☆ (Supplier assumes: – Customs clearance – VAT/GST payment – Local certification fees) |
Clinics & new distributors: – Eliminates import knowledge gap – Predictable landed cost – Required for EU MDR Article 31 compliance |
2026 Critical Update: All shipments must include carbon footprint documentation per EU CBAM regulations. DDP contracts should specify supplier’s responsibility for emissions reporting.
Verified Partner Spotlight: Shanghai Carejoy Medical Co., LTD
Why Carejoy Meets 2026 Sourcing Requirements:
- Regulatory Excellence: Direct access to 19 years of audit trails; CE certificates issued by TÜV Rheinland (NB 0123) with product-specific Annex IX documentation for all X-ray systems
- MOQ Flexibility: Tiered pricing from 2 units (vs. industry 5+); OEM options with no MOQ for established distributors
- DDP Specialization: In-house logistics team handling DDP to 42 countries with radiation compliance documentation; 99.2% customs clearance rate (2025 data)
- 2026-Ready Infrastructure: Baoshan District factory (ISO 13485:2016 certified) with automated radiation testing lab meeting IEC 61223-3-5:2023
Contact for Technical Sourcing:
Email: [email protected] (Reference: “2026 X-Ray Sourcing Guide”)
WhatsApp: +86 15951276160 (24/7 technical support line)
Ready to Source Compliant Dental X-Ray Systems in 2026?
Request Carejoy’s 2026 Regulatory Compliance Dossier including:
• Full CE Technical File Index • DDP Cost Calculator • Radiation Safety Test Protocols
Disclaimer: This guide reflects 2026 regulatory standards. Always consult local authorities before procurement. Shanghai Carejoy is presented as a verified industry partner based on 19 years of export compliance data; inclusion does not constitute endorsement by this publication. Product specifications subject to change per IEC 60601-2-65:2026 amendments.
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Frequently Asked Questions: Meditrix X-Ray Machine Acquisition
Target Audience: Dental Clinics & Authorized Equipment Distributors
| Question | Answer |
|---|---|
| 1. What is the required input voltage and power compatibility for the Meditrix X-Ray Machine in 2026, and is it suitable for international clinic installations? | The Meditrix X-Ray System operates on a standard input voltage of 100–240 VAC, 50/60 Hz, making it compatible with global electrical standards. It features an auto-switching power supply, ensuring seamless integration across regions including North America, Europe, Asia, and the Middle East. A dedicated 15A circuit is recommended for optimal performance and compliance with IEC 60601-1 safety standards. |
| 2. Are spare parts for the Meditrix X-Ray Machine readily available, and what is the lead time for critical component replacement? | Yes, all critical spare parts—including X-ray tubes, sensors, collimators, and control boards—are maintained in regional distribution hubs across North America, EMEA, and APAC. As of 2026, Meditrix guarantees 95% part availability with standard lead times of 3–5 business days for in-stock components. Distributors receive priority access through the Meditrix Partner Portal, enabling same-week fulfillment for contracted service networks. |
| 3. What does the installation process for the Meditrix X-Ray Machine involve, and is on-site technical support provided? | Installation includes site assessment, wall mounting (for fixed models), electrical validation, network integration, and calibration. Meditrix provides certified technician-led installation as part of the purchase agreement. On-site support is standard for clinic setups, with remote diagnostics available pre-installation. Turnkey deployment typically takes 4–6 hours, with full compliance documentation delivered post-installation. |
| 4. What warranty coverage is included with the Meditrix X-Ray Machine, and are extended service plans available? | The Meditrix X-Ray Machine comes with a comprehensive 3-year limited warranty covering parts, labor, and X-ray tube performance. Extended service agreements (ESA) are available up to 7 years, including predictive maintenance, software updates, and priority dispatch. Distributors may offer bundled warranty options tailored to clinic volume and usage tiers. |
| 5. How does Meditrix ensure continued support for spare parts and service beyond 2030, given long-term clinic equipment lifecycle planning? | Meditrix adheres to ISO 13485 lifecycle management protocols, guaranteeing spare part availability for a minimum of 10 years post-discontinuation of any model. As of 2026, the current Meditrix X-Ray series is projected for active support through 2036. Firmware and compatibility updates will be provided via secure cloud portal, ensuring integration with evolving clinic IT infrastructure. |
Note: Specifications and service terms are subject to change. For the latest technical documentation and distributor-specific agreements, consult the official Meditrix Partner Resource Center.
Need a Quote for Meditrix X Ray Machine?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


