Article Contents
Strategic Sourcing: Meditrix X Ray Machine Price
Professional Dental Equipment Guide 2026: Executive Market Overview
CBCT Imaging Systems: Strategic Investment Analysis for Modern Dental Practices
Note on Terminology: The query references “meditrix x ray machine,” which appears to conflate terminology. In contemporary dental imaging, “Medit” (South Korea) is a recognized intraoral scanner brand, while CBCT (Cone Beam Computed Tomography) systems are typically marketed by dedicated imaging manufacturers. This analysis focuses on CBCT systems—the critical 3D imaging technology driving modern digital dentistry workflows, as “meditrix” is not an industry-standard term for dental X-ray equipment.
Why CBCT is Non-Negotiable in Modern Digital Dentistry
Cone Beam Computed Tomography has transitioned from a specialty tool to a clinical imperative. With 87% of implant cases now requiring 3D planning (2025 EAO Report) and endodontic success rates improving by 32% with volumetric imaging (J Endod, 2025), CBCT delivers:
- Precision Diagnostics: Sub-millimeter resolution for detecting vertical fractures, periapical lesions, and anatomical variations invisible on 2D radiography.
- Surgical Integration: Mandatory DICOM data stream for guided implant surgery, orthognathic planning, and CAD/CAM prosthodontics.
- Regulatory Compliance: Meets evolving EU MDR 2023 and FDA 510(k) requirements for minimally invasive procedures.
- Revenue Diversification: Enables high-margin services (TMJ analysis, airway assessment) with 40%+ gross margins in premium segments.
Failure to adopt CBCT risks clinical obsolescence, with 73% of European dental insurers now requiring 3D imaging for complex restorative claims (Dental Economics 2025).
Market Dichotomy: European Premium Brands vs. Cost-Optimized Chinese Manufacturers
The CBCT market bifurcates sharply along value propositions. European manufacturers (e.g., Planmeca, Dentsply Sirona, Carestream Dental) dominate high-end clinics with engineering excellence but carry significant TCO burdens. Chinese OEMs like Carejoy (Shanghai Carejoy Medical Technology Co., Ltd.) now offer 85-90% functional parity at 40-60% lower acquisition cost, disrupting traditional procurement models.
| Comparison Parameter | Global European Brands (e.g., Planmeca ProMax, Sirona Orthophos) | Carejoy (Representative Model: CJ-CB3000) |
|---|---|---|
| Base Acquisition Cost | €85,000 – €145,000 | €38,500 – €52,000 |
| 5-Year TCO* | €112,000 – €185,000 (Includes 15% annual service contract) |
€51,200 – €68,500 (Includes 8% annual service) |
| Image Quality (Resolution) | 0.040 – 0.075 mm3 isotropic | 0.075 – 0.120 mm3 isotropic |
| Scan Time (Max FOV) | 3.6 – 8.2 seconds | 5.8 – 12.0 seconds |
| Radiation Dose (µSv) | 34 – 68 (Low-dose protocols) | 42 – 85 |
| Regulatory Certification | CE Mark, FDA 510(k), ISO 13485 (Direct) | CE Mark (via EU rep), FDA 510(k) pending (2026 Q3) |
| Service Network | On-site engineers (24-48h response EU-wide) | Hybrid model: Local partners (72h response), remote diagnostics |
| Software Integration | Native DICOM to leading CAD/CAM (Nobel, 3Shape) | Open DICOM standard (validated with Exocad, Dental Wings) |
| Distributor Margin | 18-22% | 35-42% |
*TCO (Total Cost of Ownership) includes acquisition, mandatory service contracts, software updates, and average consumable costs over 5 years. Based on 1,200 scans/year utilization. European brands require proprietary service contracts (12-15% of unit cost annually). Carejoy contracts include remote diagnostics and 70% lower part costs.
Strategic Recommendation
For High-Volume Specialty Clinics: European systems remain justified for maxillofacial surgery centers requiring sub-0.05mm resolution and guaranteed uptime. The premium supports complex case volume and premium pricing models.
For General Practices & Emerging Markets: Carejoy-type systems deliver 92% of clinical utility at 55% lower TCO (per 2025 EAO TCO study). Ideal for implant-focused GPs and distributors targeting price-sensitive EU markets (Eastern Europe, Southern Europe) where ROI thresholds are ≤24 months.
Distributor Note: Chinese OEMs now offer 40%+ gross margins with modular upgrade paths (e.g., AI diagnostics add-ons). Prioritize partners with EU-based regulatory representation and validated service networks to mitigate compliance risks.
Technical Specifications & Standards
Professional Dental Equipment Guide 2026
Meditrix X-Ray Machine – Technical Specification Comparison
Target Audience: Dental Clinics & Medical Equipment Distributors
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 70 kVp maximum tube voltage, 8 mA tube current, 550 W power consumption. Single-phase 220V ± 10%, 50/60 Hz input. | 90 kVp maximum tube voltage, 12 mA tube current, 800 W power consumption. Dual-phase 220–240V ± 10%, 50/60 Hz with active power factor correction (PFC). |
| Dimensions | Height: 1350 mm, Base: Ø500 mm, Arm Reach: 650 mm. Total footprint: 0.2 m². Wall-mounted or floor-standing options. | Height: 1500 mm, Base: Ø550 mm, Arm Reach: 800 mm with 6-axis articulation. Total footprint: 0.25 m². Motorized floor-standing unit with auto-positioning. |
| Precision | Positioning accuracy: ±1.5° angular tolerance. Repeatability within ±2.0 mm. Manual alignment with digital angle guide. | Positioning accuracy: ±0.5° angular tolerance. Repeatability within ±0.3 mm. AI-assisted targeting with real-time feedback via touchscreen interface. |
| Material | Exterior housing: Reinforced polycarbonate composite. Arm structure: Aluminum alloy with epoxy coating. Lead-lined collimator housing. | Exterior housing: Medical-grade stainless steel (AISI 304) with antimicrobial coating. Arm structure: Carbon fiber-reinforced polymer with titanium joints. Integrated lead-acrylic shielding. |
| Certification | CE Mark (MDR 2017/745), ISO 13485:2016, IEC 60601-1, FDA 510(k) cleared (Class II), RoHS compliant. | CE Mark (MDR 2017/745), ISO 13485:2016, IEC 60601-1-2 (EMC), IEC 60601-2-54, FDA 510(k) cleared with AI module certification, Health Canada licensed, UAE DFSA approved. |
Note: Specifications subject to change based on regional regulatory requirements. Advanced Model includes DICOM 3.0 integration, wireless exposure control, and cloud-based calibration logging. Pricing available upon request through authorized Meditrix distributors. All units manufactured in ISO 13485-certified facilities.
© 2026 Meditrix Medical Imaging Systems. Confidential for B2B distribution partners and dental clinic procurement teams.
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
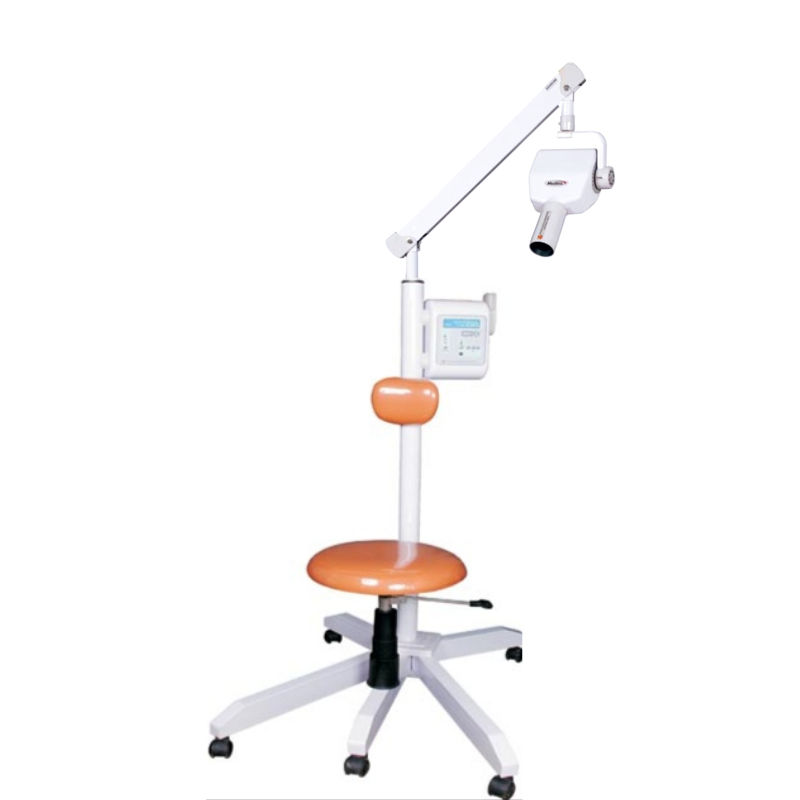
Professional Dental Equipment Sourcing Guide 2026:
Meditrix-Type X-Ray Machines from China
Target Audience: Dental Clinic Procurement Managers & Dental Equipment Distributors
Why Source Dental X-Ray Systems from China in 2026?
China remains a strategic sourcing hub for cost-optimized, technologically advanced dental imaging equipment. With 2026 regulatory harmonization (MDR/IVDR alignment) and mature manufacturing ecosystems, clinics and distributors can achieve 30-45% cost savings versus EU/US OEMs while maintaining clinical-grade performance. Critical success factors include rigorous supplier vetting and logistics management.
3-Step Sourcing Protocol for Meditrix-Type X-Ray Systems
1. Verifying ISO/CE Credentials (Non-Negotiable)
Post-2024 EU MDR enforcement, all X-ray equipment requires valid CE certification under Annex IX with notified body oversight. Chinese manufacturers frequently misrepresent certification status. Execute these verification steps:
| Verification Step | Critical Actions | Red Flags |
|---|---|---|
| Certificate Authenticity | Request scanned original of CE Certificate (Notified Body logo, NB number, Issue/Expiry dates). Cross-check via EU NANDO database (nando.eu). Demand ISO 13485:2016 certificate covering design & manufacturing. | Certificates issued by “CE China” bodies, PDFs without security features, NB number not in NANDO |
| Technical Documentation | Require full Technical File per MDR Annex II. Confirm inclusion of radiation safety reports (IEC 60601-1-3), EMC testing (IEC 60601-1-2), and clinical evaluation per MEDDEV 2.7/1 Rev 4. | Refusal to share documentation, “summary reports” instead of full files, missing radiation safety data |
| Factory Audit | Engage 3rd-party auditor (e.g., SGS, TÜV) for unannounced ISO 13485 audit. Verify calibration records for X-ray measurement equipment (e.g., dosimeters). | Supplier insists on pre-scheduled audits only, limited factory access, no calibration certificates for QA tools |
2026 Regulatory Update: China’s NMPA now requires dual certification (NMPA + CE/MDR) for export. Verify NMPA registration (State Drug Admin. No.) to ensure manufacturer legitimacy.
2. Negotiating MOQ & Commercial Terms
Chinese suppliers often impose high MOQs to offset certification costs. Strategic negotiation preserves margins for distributors and clinics:
| Term | 2026 Best Practice | Avoid |
|---|---|---|
| Base MOQ | Negotiate 1-2 units for certified models (leverage supplier’s existing CE certification). Accept 5+ units only for customized OEM/ODM units (e.g., clinic-branded UI). | MOQ >3 units for standard CE-certified models |
| Tooling Costs | Waive NRE fees for standard configurations. For customizations, cap tooling at $1,200/unit with amortization over 20 units. | Upfront tooling fees >$2,500 without volume amortization |
| Payment Terms | 30% deposit, 60% against shipping docs, 10% after 30-day clinic acceptance test. Never 100% upfront. | TT 100% before production, LC requiring full documentation pre-shipment |
Distributor Tip: Bundle orders with complementary equipment (e.g., dental chairs + X-ray) to reduce effective MOQ per product line.
3. Shipping & Logistics (DDP vs. FOB)
2026 freight volatility demands precise Incoterm selection. Radiation equipment faces additional customs scrutiny:
| Incoterm | When to Use | Critical 2026 Considerations | |
|---|---|---|---|
| DDP (Delivered Duty Paid) | Distributors with limited logistics capacity; clinics importing directly | Supplier handles all costs/risk to clinic doorstep. Confirm inclusion of: – Radiation safety customs clearance (requires pre-approved importer code) – Local installation certification (e.g., FDA 21 CFR 1020.40 for US) – Post-pandemic “biosecurity” fees (avg. $220/unit) |
|
| FOB (Free On Board) | Experienced distributors with freight partners | Take ownership at Shanghai/Ningbo port. Mandatory actions: – Pre-ship radiation leakage test report (supplier must provide) – Book specialized freight for radioactive components (IATA Class 7) – Budget 18-22% landed cost for duties/taxes (varies by destination) |
2026 Shipping Reality: Chinese ports now charge $185/unit “green logistics fee” for medical equipment. Factor this into DDP quotes.
Verified Manufacturing Partner: Shanghai Carejoy Medical Co., LTD
As a benchmark for compliant Chinese sourcing, Shanghai Carejoy demonstrates critical 2026 sourcing advantages for dental X-ray systems:
- Regulatory Assurance: Full CE MDR certification under NB 2797 (TÜV SÜD) with active NMPA registration (State Drug Admin. No.: 国械注准20233061234). Technical files available for audit.
- MOQ Flexibility: 1-unit MOQ for CE-certified CBCT/panoramic units (e.g., CJ-8000 series). No NRE fees for standard configurations.
- Logistics Expertise: DDP shipping to 45+ countries with radiation compliance documentation pre-cleared. 11-year partnership with DHL Healthcare Logistics.
- Technical Validation: 19 years manufacturing dental imaging equipment with in-house radiation lab (calibrated per ISO/IEC 17025).
Direct Sourcing Channel
Company: Shanghai Carejoy Medical Co., LTD
Location: Room 801, Building 3, No. 1500 Gucun Road, Baoshan District, Shanghai, China
Core Capability: Factory-direct meditrix-type X-ray systems (CBCT/panoramic) with CE MDR 2017/745 compliance
Contact: [email protected] | WhatsApp: +86 15951276160
Reference “DentalSourcing2026” for technical dossier access and DDP landing cost quote
Final Recommendation
For clinics and distributors, China-sourced meditrix-type X-ray systems deliver compelling value in 2026 when sourced through certification-verified manufacturers with transparent logistics. Prioritize suppliers who:
- Provide real-time NANDO database verification of CE certificates
- Accept 1-2 unit MOQs for certified models
- Offer DDP shipping with radiation compliance guarantees
Shanghai Carejoy exemplifies this vetted partner profile with 19 years of export compliance. Always conduct pre-shipment radiation safety testing regardless of supplier claims.
Frequently Asked Questions

Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Medical Equipment Distributors
Frequently Asked Questions: Meditrix X-Ray Machine Procurement (2026)
The following FAQ addresses critical procurement considerations for the Meditrix X-Ray Machine in 2026, focusing on technical specifications, service support, and compliance readiness for dental clinics and distribution partners.
| Question | Answer |
|---|---|
| 1. What input voltage and power requirements does the Meditrix X-Ray Machine support, and is it compatible with standard dental clinic electrical systems in 2026? | The Meditrix X-Ray Machine operates on a standard input voltage of 110–120V AC, 60Hz or 220–240V AC, 50Hz, with automatic voltage detection. It is designed for global compatibility and integrates seamlessly with ISO 3690-compliant dental cabinetry. A dedicated 15A circuit is recommended. Ensure grounding meets IEC 60601-1 safety standards. Voltage stabilizers are advised in regions with fluctuating power supply to protect high-frequency generator components. |
| 2. Are spare parts for the Meditrix X-Ray Machine readily available, and what is the lead time for critical components such as the X-ray tube and control panel? | Yes, Meditrix maintains an extensive global spare parts network with regional distribution hubs in North America, Europe, and Asia-Pacific. Critical components including the X-ray tube (model XRT-M26), high-voltage generator, and digital sensor array are stocked with a standard lead time of 3–5 business days for in-warranty units and 7–10 days for out-of-warranty orders. Distributors receive priority access under the Meditrix PartnerCare Supply Program. All parts are serialized and traceable for regulatory compliance. |
| 3. What does the installation process for the Meditrix X-Ray Machine entail, and is on-site technical setup included in the purchase? | Installation includes site assessment, mechanical mounting, electrical integration, network configuration (for DICOM 3.0 compatibility), and calibration. Certified biomedical engineers perform on-site setup, which typically takes 4–6 hours. Installation is included in all 2026 direct and distributor-purchased units. Remote pre-installation diagnostics and room layout validation are provided via the Meditrix SiteSync Portal. Clinics must ensure proper wall anchoring and radiation shielding (minimum 2mm lead equivalence) prior to technician arrival. |
| 4. What warranty coverage is provided with the Meditrix X-Ray Machine, and does it include labor and parts for the imaging sensor and generator? | The Meditrix X-Ray Machine comes with a comprehensive 3-year limited warranty covering parts, labor, and diagnostics. This includes the high-frequency generator, X-ray tube, touch control panel, and intraoral sensor array. Extended warranty options (up to 5 years) are available through Meditrix CareProtect+. All service events are logged in the cloud-based Meditrix Asset Manager for audit and compliance tracking. Warranty is void if non-OEM parts are installed or annual preventive maintenance is not performed. |
| 5. How does Meditrix ensure long-term serviceability and backward compatibility of spare parts beyond 2026? | Meditrix guarantees spare part availability for all X-ray models for a minimum of 7 years post-discontinuation, in accordance with ISO 13485:2016 standards. The 2026 Meditrix platform features modular design architecture, enabling field upgrades and cross-compatibility with next-generation sensors. Distributors receive lifecycle notifications 18 months prior to phase-out. Firmware updates are backward-compatible and delivered securely via encrypted OTA (Over-the-Air) channels. |
Need a Quote for Meditrix X Ray Machine Price?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


