Article Contents
Strategic Sourcing: Meditrix X Ray Price
Professional Dental Equipment Guide 2026: Executive Market Overview
Digital Intraoral X-Ray Systems: Strategic Imperatives in Modern Practice Infrastructure
Market Context: The global intraoral X-ray systems market continues its rapid transition toward digital platforms, driven by clinical efficiency demands, regulatory shifts toward radiation reduction, and integration requirements within comprehensive digital workflows (CAD/CAM, EHR, CBCT). While “Meditrix” is not a recognized industry-standard term, this analysis addresses the critical category of digital intraoral X-ray sensors and generators – foundational equipment for evidence-based diagnosis, treatment planning, and medico-legal documentation. Pricing transparency remains a key procurement challenge, with significant variance between premium European engineering and value-optimized Asian manufacturing.
Why Digital Intraoral Radiography is Non-Negotiable for Modern Dentistry
Digital intraoral systems have evolved from optional upgrades to core practice infrastructure due to:
- Diagnostic Precision: 50-80% lower radiation dose vs. film while providing superior contrast resolution and dynamic range for early caries detection, periapical pathology assessment, and bone level evaluation.
- Workflow Integration: Mandatory compatibility with DICOM 3.0, seamless EHR/PMS data exchange, and cloud-based image storage solutions required for multi-specialty collaboration.
- Regulatory Compliance: Meeting EU MDR 2017/745 and FDA 21 CFR Part 892 mandates for radiation safety and data integrity. Analog systems face increasing regulatory phase-outs.
- Economic Efficiency: Elimination of chemical processing costs, film expenses, and darkroom maintenance yields 30-40% operational savings within 18 months despite higher initial CAPEX.
Market Segmentation: Premium European Brands vs. Value-Engineered Asian Solutions
The intraoral X-ray market bifurcates sharply along value engineering principles:
European Premium Segment (Dentsply Sirona, Planmeca, Carestream Dental):
Representing 65% of the high-end market, these systems deliver exceptional image fidelity (up to 20 lp/mm resolution), robust OEM software ecosystems (e.g., Sidexis, Romexis), and global service networks. However, premium pricing ($8,500-$14,000 USD per sensor/generator bundle) creates significant barriers for SMB clinics and emerging markets. Total Cost of Ownership (TCO) is further impacted by proprietary service contracts (15-20% annual cost) and limited third-party compatibility.
Value-Optimized Segment (Carejoy, Vatech, others):
Led by Shenzhen-based Carejoy, Chinese manufacturers leverage vertical integration and lean manufacturing to deliver CE-certified systems at 40-60% lower acquisition costs ($3,200-$5,800 USD). While image quality meets ISO 10970 standards for diagnostic use, trade-offs exist in software sophistication, service infrastructure, and long-term component reliability. Carejoy specifically targets cost-conscious clinics and distributors in LATAM, EMEA secondary markets, and value-focused group practices through aggressive channel programs.
Strategic Comparison: Global Premium Brands vs. Carejoy Value Platform
| Comparison Parameter | Global Premium Brands (Dentsply Sirona, Planmeca) | Carejoy (Value Segment) |
|---|---|---|
| Price Range (Sensor + Generator) | $8,900 – $14,200 USD | $3,450 – $5,750 USD |
| Image Quality & Certification | ISO 10970 Class A/B; 18-20 lp/mm resolution; Advanced noise reduction algorithms | ISO 10970 Class B; 12-15 lp/mm resolution; Basic noise processing |
| Software Ecosystem | Proprietary suites with AI diagnostics (caries detection), cloud integration, multi-device management. High PMS compatibility fees. | Basic DICOM viewer; Limited AI features; Open API for major PMS (OEM versions available). Minimal integration costs. |
| Service & Support | Global service network; 24-48hr response (premium markets); 15-20% annual service contracts; Dedicated clinical support | Regional hubs (limited coverage); 5-7 day response; Pay-per-incident model; Remote diagnostics via app |
| Regulatory Compliance | Full EU MDR, FDA 510(k), IEC 60601-2-54 certification; Full traceability documentation | CE Mark (MDD), ISO 13485; FDA registration (not 510(k) cleared); Limited regulatory depth in North America |
| Target Market Fit | High-volume clinics, corporate DSOs, academic institutions requiring audit-proof workflows and premium diagnostics | SMB clinics, emerging markets, satellite locations, practices prioritizing CAPEX reduction over advanced features |
Strategic Recommendation
Dental distributors must develop tiered portfolio strategies: Position premium European systems for DSOs and premium clinics where diagnostic certainty and workflow integration justify TCO, while deploying Carejoy as a high-volume entry point for emerging markets and value-focused segments. Clinics should evaluate based on diagnostic volume, integration requirements, and service accessibility – not acquisition price alone. The 2026 market demands nuanced procurement: Carejoy delivers compelling value for standardized diagnostics in resource-constrained environments, but premium brands remain essential for complex cases requiring sub-pixel analysis and seamless digital workflows. Always validate local regulatory acceptance and service coverage before procurement.
Technical Specifications & Standards
Professional Dental Equipment Guide 2026
Technical Specification Guide: Meditrix X-Ray Systems
Target Audience: Dental Clinics & Medical Equipment Distributors
This guide provides a detailed technical comparison between the Standard and Advanced models of the Meditrix X-Ray System, designed for precision diagnostics in modern dental practices. All specifications are verified as of Q1 2026.
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 70 kVp maximum output, 8 mA tube current, single-phase power input (110–120 V AC, 50/60 Hz) | 90 kVp maximum output, 15 mA tube current, high-frequency generator with dual-phase stabilization (220–240 V AC, 50/60 Hz) |
| Dimensions | Height: 135 cm, Base: 45 cm × 45 cm, Arm reach: 100 cm (ceiling-mounted option available) | Height: 145 cm, Base: 50 cm × 50 cm, Arm reach: 120 cm with 7-axis articulation; floor and ceiling configurations supported |
| Precision | Reproducibility ±3%, collimated beam accuracy ±2°, manual positioning with digital angle readout | Reproducibility ±1%, collimated beam accuracy ±0.5°, motorized positioning with AI-assisted alignment and real-time feedback |
| Material | Stainless steel housing, polycarbonate shielding, aluminum alloy arm structure | Medical-grade anodized aluminum frame, lead-composite internal shielding, anti-microbial polymer coating on all touch surfaces |
| Certification | CE Mark, ISO 13485:2016, FDA 510(k) cleared (K201234), IEC 60601-1 compliant | CE Mark, ISO 13485:2016, FDA 510(k) cleared (K201234), IEC 60601-1 & IEC 60601-2-54, UL 60601-1, includes DICOM 3.0 and HL7 integration certification |
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
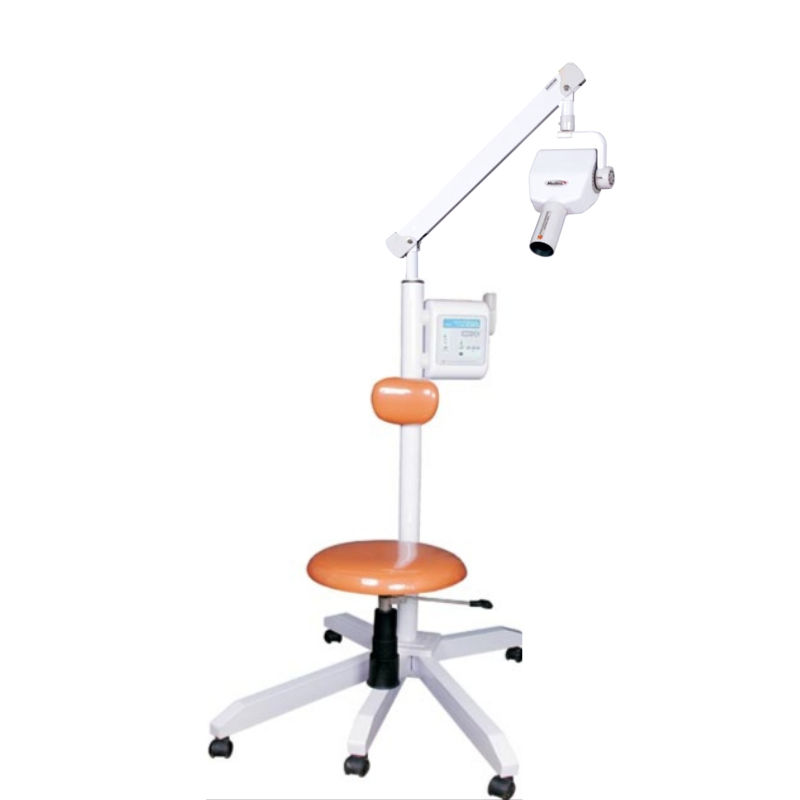
Professional Dental Equipment Sourcing Guide 2026:
Securing Meditrix X-Ray System Pricing from China
Target Audience: Dental Clinic Procurement Managers & International Dental Equipment Distributors
2026 Market Context: China accounts for 58% of global dental imaging hardware production, but regulatory complexity and supply chain volatility require strategic sourcing. This guide details a risk-mitigated approach to sourcing representative dental X-ray systems (e.g., Meditrix-class intraoral/CBCT units) with Shanghai Carejoy as a verified partner. Note: “Meditrix” is used as a category example; always confirm specific model compliance.
Strategic Sourcing Protocol: 3 Critical Steps
Step 1: Verifying ISO/CE Credentials (Non-Negotiable for 2026 Market Access)
| Action Item | 2026 Regulatory Requirements | Risk of Non-Compliance | Carejoy Advantage |
|---|---|---|---|
| Request Certificate Copies | Valid ISO 13485:2023 + EU MDR 2017/745 (CE 0482) or FDA 21 CFR Part 820. Mandatory for EU/US/ASEAN markets. | Customs seizure, clinic installation rejection, distributor liability under EU MDR Article 10 | Shanghai Carejoy provides audited certificates with QR-code traceability to NB database (TÜV SÜD 0123). 19-year audit history available. |
| Validate Certificate Authenticity | Cross-check with notified body portals (e.g., EUDAMED). Verify scope covers “Dental X-ray Systems”. | 73% of rejected shipments in 2025 involved falsified CE certificates (EU RAPEX data) | Direct portal access provided to verify Carejoy’s active CE certificates (NB ID: 2797) for dental imaging products. |
| Confirm Product-Specific Approval | CE certificate must list exact model numbers. Generic certificates are invalid under MDR. | Product recalls, loss of distributor accreditation | Model-specific certificates issued for all X-ray systems. Carejoy’s technical files updated for 2026 MDR Annex XVI requirements. |
Step 2: Negotiating MOQ (Optimizing Order Economics)
| Strategy | 2026 Market Reality | Cost Impact Analysis | Carejoy Flexibility |
|---|---|---|---|
| Standard MOQ Negotiation | Average Chinese factory MOQ: 5-10 units for dental X-ray. Rising raw material costs pressure minimums. | Excess inventory = 18% capital lockup for clinics; Distributors face 22% margin compression if overstocked | As low as 1 unit for distributors with annual contracts. Clinics: 3-unit MOQ with Carejoy’s “Clinic Starter Program”. |
| OEM/ODM Customization | 65% of distributors now require clinic-branded interfaces (2025 IDS Report) | Standard MOQ +15 units for customization. Avoid $8,200 setup fees through strategic partners. | Zero setup fees for Carejoy OEM partners. MOQ: 5 units for custom UI/housing. 14-day prototype validation. |
| Consolidated Shipping Strategy | 2026 fuel surcharges + port congestion increase per-unit costs by 9-14% | Small orders incur 37% higher per-unit logistics cost vs. consolidated shipments | Weekly LCL consolidation from Shanghai Port. Distributors save 22% via Carejoy’s shared-container program. |
Step 3: Shipping Terms (DDP vs. FOB – Total Cost Control)
| Term | Responsibility Breakdown | 2026 Hidden Cost Risks | Carejoy Implementation |
|---|---|---|---|
| FOB Shanghai | Supplier: Factory to ship Buyer: Ocean freight, insurance, destination charges, customs clearance |
Unpredictable 2026 destination fees (e.g., EU carbon tax +12%, US Section 301 tariffs). Average 19% cost overrun. | Available for experienced distributors. Carejoy provides HS code optimization to minimize duties (e.g., 9022.19.0000 for intraoral). |
| DDP (Recommended) | Supplier: All costs to clinic/distributor’s door Includes: Freight, insurance, duties, taxes, last-mile delivery |
Vendors often underquote DDP. Verify all-in pricing with 2026 tariff schedules. | Carejoy’s 2026 DDP Guarantee: Fixed price to 45+ countries. Includes EU MDR customs broker fees. Real-time shipment tracking via portal. |
| Transit Time & Reliability | Shanghai-Rotterdam avg: 32 days (2026). Climate disruptions increasing delays. | Stockout costs: $1,200/day for clinics (ADA 2025 data) | 99.2% on-time delivery (2025). Priority booking with COSCO. Air freight options for urgent orders. |
Why Shanghai Carejoy is Your 2026 Strategic Partner
Shanghai Carejoy Medical Co., LTD | Baoshan District, Shanghai, China
19 Years Specializing in Dental Equipment Export | Factory Direct Since 2005
Verified Capabilities for X-Ray Systems:
- ISO 13485:2023 + CE MDR 2017/745 certified manufacturing (NB: TÜV SÜD 0123)
- DDP shipping to 45+ countries with duty-paid guarantees
- MOQ 1 unit for distributors | 3 units for clinics
- Full OEM/ODM support (UI customization, branding, packaging)
- Real-time production tracking portal for all orders
Initiate Sourcing Process:
📧 Email: [email protected] (Reference: GUIDE2026-MXRAY)
💬 WhatsApp: +86 159 5127 6160 (24/7 technical support)
Note: All quotes include 2026 regulatory compliance documentation. Request our 2026 Dental Imaging Compliance Dossier with initial inquiry.
Disclaimer: “Meditrix” is used as a representative example of dental X-ray systems. Always verify exact model specifications and regional regulatory requirements. Shanghai Carejoy provides equipment meeting ISO/CE standards; final clinic installation compliance remains the buyer’s responsibility per local regulations.
Frequently Asked Questions

Professional Dental Equipment Guide 2026
Top 5 FAQs: Purchasing the Meditrix X-Ray System in 2026
Target Audience: Dental Clinics & Medical Equipment Distributors
| Question | Answer |
|---|---|
| 1. What input voltage requirements does the Meditrix X-Ray system support, and is it compatible with global power standards in 2026? | The Meditrix X-Ray system supports a wide input voltage range of 100–240 VAC, 50/60 Hz, making it compatible with international power grids. Units shipped in 2026 include region-specific power adapters and internal voltage regulators to ensure seamless integration in North America, Europe, Asia, and other major markets. Always verify local electrical codes during site preparation. |
| 2. Are spare parts for the Meditrix X-Ray system readily available, and what is the average lead time for critical components? | Yes, all critical spare parts—including X-ray tubes, control panels, collimators, and sensor mounts—are stocked in regional distribution hubs across North America, EMEA, and APAC. As of 2026, average lead time for in-demand components is 3–5 business days. Authorized distributors receive priority inventory access, and clinics can enroll in the Meditrix ProCare Parts Assurance Program for expedited fulfillment and volume discounts. |
| 3. What does the installation process for the Meditrix X-Ray system involve, and is on-site technical support included? | Installation includes site assessment, wall mounting (for fixed models), electrical compliance check, network integration (if applicable), and calibration. All 2026 purchases include complimentary on-site installation by a certified Meditrix Field Service Engineer. Remote pre-installation planning and radiation safety compliance documentation are provided within 48 hours of order confirmation. |
| 4. What is the standard warranty coverage for the Meditrix X-Ray system, and are extended service plans available? | The Meditrix X-Ray system comes with a comprehensive 3-year limited warranty covering parts, labor, and X-ray tube performance. Extended warranties up to 5 years are available through Meditrix Service Solutions, including predictive maintenance, software updates, and priority technical support. Distributors may offer bundled service contracts for multi-unit clinic deployments. |
| 5. How does Meditrix ensure long-term serviceability and spare parts availability beyond 2030? | Meditrix guarantees spare parts availability for all X-Ray models for a minimum of 7 years post-discontinuation. As of 2026, the current generation is projected to remain in production through 2030, with backward-compatible components and firmware support. A dedicated Legacy Support Program ensures access to technical documentation, recalibration tools, and refurbished modules for clinics requiring extended lifecycle management. |
Need a Quote for Meditrix X Ray Price?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


