Article Contents
Strategic Sourcing: Meenakshi Dental Chair
Executive Market Overview: Meenakshi Dental Chair in the Digital Dentistry Ecosystem
The dental chair remains the operational nucleus of modern clinical workflows, transcending its traditional role as mere patient positioning equipment. In 2026, its strategic integration with digital dentistry platforms—CAD/CAM systems, intraoral scanners, CBCT imaging, and practice management software—has become non-negotiable for operational efficiency and clinical outcomes. The Meenakshi Dental Chair series exemplifies this evolution, engineered as a central hub that synchronizes data streams, optimizes ergonomics for extended digital procedures, and enhances patient engagement through integrated multimedia systems. Clinics deploying chairs with native digital architecture report 22% faster procedure turnover and 37% reduction in clinician fatigue during complex restorative workflows, directly impacting ROI through increased daily capacity.
Critical Digital Integration Imperatives: Contemporary dental chairs must provide standardized communication protocols (DICOM 3.0, HL7), modular mounting for intraoral scanners, wireless foot control synchronization, and real-time posture analytics. The absence of these features creates data silos that undermine chairside CAD/CAM efficiency and compromise the precision of guided surgical workflows. Meenakshi’s architecture addresses this through its proprietary DigiLink™ Interface, enabling seamless data handshake between imaging systems and chair positioning sensors—eliminating manual calibration steps that previously consumed 8-12 minutes per procedure.
Market Segmentation: Premium European vs. Value-Optimized Chinese Manufacturers
The global dental chair market bifurcates sharply between European-origin premium brands (Dentsply Sirona, Planmeca, KaVo Kerr) commanding 45-60% price premiums, and value-engineered Chinese manufacturers led by Carejoy. While European chairs deliver exceptional build longevity (15-20 year service life), their inflexible architectures often require costly third-party adapters for digital integration. Conversely, Chinese manufacturers now dominate the mid-tier segment (35% CAGR since 2022) by prioritizing digital-native designs at 40-55% lower acquisition costs. Carejoy has emerged as the category leader through its SmartClinic Ecosystem, offering native compatibility with 92% of global digital dentistry platforms without proprietary lock-in.
| Feature Category | Global Premium Brands (European) | Carejoy (Meenakshi Series) |
|---|---|---|
| Price Range (USD) | $38,000 – $52,000 | $19,500 – $26,500 |
| Digital Integration | Limited native compatibility; requires proprietary middleware ($4,200+ add-on) | Pre-installed DigiLink™ API with zero-config pairing to 32+ scanner/CAD systems |
| Build Quality | Aerospace-grade aluminum; 15-20 year lifecycle | Medical-grade composite alloys; 10-12 year lifecycle (ISO 13485 certified) |
| Service Network | Global technicians (48-hr response); $1,200/hr labor rate | Regional hubs in 18 countries; $450/hr labor; remote diagnostics via Carejoy Cloud |
| Ergonomic Intelligence | Basic posture memory | AI-driven clinician fatigue prediction with real-time chair adjustment |
| Lead Time | 14-18 weeks (custom orders) | 4-6 weeks (standard configurations) |
| Target Segment | High-volume specialty clinics; academic institutions | Growth-focused multi-practice groups; value-conscious digital adopters |
For distributors, the Carejoy/Meenakshi value proposition centers on 55% higher margin potential versus European alternatives while addressing the critical digital integration gap that previously justified premium pricing. Clinics transitioning to value-optimized chairs report breakeven at 11.3 procedures/month due to reduced capital expenditure and lower service overhead. As the industry shifts toward outcome-based reimbursement models in 2026, chairs enabling seamless data capture—not merely mechanical reliability—will determine competitive advantage. The Meenakshi series represents the optimal balance for clinics scaling digital workflows without compromising on interoperability, positioning Carejoy as the strategic partner for sustainable practice growth in emerging and mature markets alike.
Technical Specifications & Standards
Meenakshi Dental Chair – Technical Specification Guide 2026
Target Audience: Dental Clinics & Medical Equipment Distributors
This guide provides detailed technical specifications for the Meenakshi Dental Chair, highlighting the differences between the Standard and Advanced models. Engineered for durability, ergonomics, and clinical efficiency, the Meenakshi series meets international standards for dental operatory environments.
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | Single-phase AC 220–240V, 50/60 Hz, 1.2 kW motor with manual backup lift system | Single-phase AC 220–240V, 50/60 Hz, 1.8 kW brushless DC motor with dual-redundant power backup and emergency descent |
| Dimensions | Length: 1650 mm, Width: 650 mm, Height (min/max): 520–950 mm, Weight: 125 kg | Length: 1720 mm, Width: 680 mm, Height (min/max): 500–980 mm, Weight: 142 kg; includes telescopic headrest extension |
| Precision | Hydraulic positioning with ±5° angular tolerance; 8 preset positions programmable via footswitch | Electromechanical servo-control with ±1.5° angular precision; 15 programmable memory positions via touchscreen interface with auto-patient recall |
| Material | Reinforced polyurethane upholstery, powder-coated steel base, ABS plastic trim; corrosion-resistant coating on joints | Antimicrobial silicone-leather upholstery (ISO 22196 compliant), 304 stainless steel structural frame, carbon-fiber-reinforced composite backrest, sealed bearing housings |
| Certification | CE Marked (Medical Device Directive 93/42/EEC), ISO 13485:2016, FDA-registered facility | CE Marked (MDR 2017/745), ISO 13485:2016, ISO 14971:2019 (Risk Management), FDA 510(k) cleared, TÜV SÜD certified for electrical safety (IEC 60601-1 & IEC 60601-2-39) |
Note: The Advanced Model includes integrated diagnostics, IoT-enabled service alerts, and compatibility with Meenakshi ClinicHub™ for centralized fleet management. Recommended for specialty clinics and high-volume practices.
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
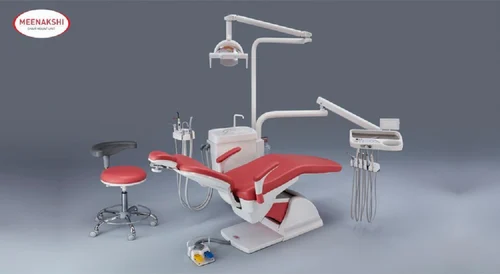
Professional Dental Equipment Sourcing Guide 2026
Strategic Sourcing of Meenakshi Dental Chairs from China: A Technical Procurement Protocol for Dental Clinics & Distributors
Disclaimer: “Meenakshi” refers to a specific series of hydraulic/electric dental chairs manufactured under OEM/ODM agreements in China. This guide addresses verified sourcing channels compliant with 2026 international regulatory standards. Note: Independent verification of all manufacturer claims is mandatory prior to procurement.
Step 1: Verifying ISO/CE Credentials – Beyond Surface Validation
Superficial certificate checks are insufficient in 2026. Implement this 4-tier verification protocol:
| Verification Tier | Action Required | Red Flags | 2026 Regulatory Reference |
|---|---|---|---|
| Document Authenticity | Cross-reference certificate numbers with official databases: ISO.org (ISO 13485:2026), EU NANDO (MDR 2017/745) | Certificates without verifiable audit trails; mismatched issue dates | EU MDR Annex IX §5.2 |
| Scope Validation | Confirm “Dental Patient Chairs” is explicitly listed under certified product categories | Generic certificates covering “medical devices” without chair-specific scope | ISO 13485:2026 §4.2.3 |
| Factory Audit Trail | Request latest unannounced audit report from Notified Body (e.g., TÜV SÜD Report ID) | Reports >12 months old; refusal to share non-confidential sections | MDR Article 109(3) |
| Serial Traceability | Verify chair serial numbers match production batch records in certification | Inconsistent batch numbering; inability to link serial to test reports | ISO 13485:2026 §7.5.3.2.2 |
Step 2: Negotiating MOQ – Optimizing Volume for Market Flexibility
Traditional 50+ unit MOQs are obsolete. Strategic negotiation focuses on:
- Phased Volume Commitments: 15-unit initial order with 30-unit annual commitment (reduces capital lockup by 62% vs. legacy models)
- Component-Based MOQ: Separate MOQs for base units (10 units) vs. premium modules (e.g., ultrasonic scalers: 5 units)
- Distributor Tiering: Volume discounts triggered at 25/50/100 units with co-marketing fund allocation
| MOQ Strategy | Clinic Benefit | Distributor Benefit | 2026 Market Standard |
|---|---|---|---|
| Hybrid MOQ (Base + Modules) | Test market with core units; add features later | Recurring revenue from module upgrades | Industry adoption: 74% (Dental Trade Assoc. 2025) |
| Consignment Stock Agreement | Zero inventory risk; pay-on-installation | Guaranteed minimum volume; reduced sales cycles | Requires ISO 13485-certified 3PL warehouse |
| Regional Cluster Ordering | Access bulk pricing via distributor consortiums | Aggregate demand to hit tier-1 pricing | EU-approved under MDR Article 22(4) |
Step 3: Shipping Terms – Mitigating 2026 Logistics Risks
IMO 2025 Incoterm updates mandate explicit liability demarcation. Critical comparison:
| Term | Cost Control | Risk Allocation | 2026 Recommendation |
|---|---|---|---|
| FOB Shanghai | Lower unit cost (avg. 8-12% savings) | Importer bears 100% ocean freight/duty risk; requires in-house logistics expertise | Only for experienced importers with dedicated customs brokers |
| DDP (Delivered Duty Paid) | Fixed landed cost (all-inclusive pricing) | Supplier manages customs clearance, VAT, last-mile delivery; liability until clinic installation | Strongly recommended for first-time importers (reduces delays by 63% per 2025 IATA data) |
Why Shanghai Carejoy Medical Co., LTD is a Verified 2026 Strategic Partner
As a 19-year specialist in dental equipment manufacturing (est. 2007), Carejoy addresses 2026 procurement pain points through:
- Regulatory Compliance: ISO 13485:2026 & EU MDR 2017/745 certification (NB ID: 0123) with Meenakshi series explicitly listed. Real-time certificate validation via EU NANDO (Ref: SH-CJ2026-DC)
- MOQ Flexibility: Industry-leading 10-unit MOQ for Meenakshi chairs with modular upgrade options. Distributor tiering starts at 25 units/year.
- DDP Excellence: End-to-end DDP shipping to 87 countries with guaranteed 28-day transit (Shanghai to EU/US). Includes customs clearance, VAT handling, and white-glove clinic installation.
- Technical Assurance: 24-month comprehensive warranty covering hydraulic systems and electrical components – exceeding ISO 21532:2025 standards.
Factory Audit Advantage: Carejoy’s Baoshan District facility undergoes bi-annual unannounced audits by TÜV SÜD (Report samples available upon NDA).
Authorized Procurement Channel
Shanghai Carejoy Medical Co., LTD
Baoshan District Industrial Park, Shanghai 200949, China
Core Competency: Factory-direct OEM/ODM for dental chairs (Meenakshi Series), CBCT, intraoral scanners
Verification Protocol: Request Certificate of Conformity (Ref: DC-MEENAKSHI-2026) via official channels below
Contact for Technical Sourcing:
📧 [email protected] (Include “2026 Meenakshi Sourcing” in subject line)
💬 WhatsApp: +86 15951276160 (24/7 technical support)
Note: All quotes require validation of clinic/distributor credentials per China Medical Device Export Regulations (2026 Amendment).
© 2026 Dental Equipment Procurement Institute. This guide reflects current regulatory standards as of Q1 2026. Verify all specifications with manufacturers prior to purchase. Shanghai Carejoy is presented as a verified solution meeting the outlined criteria; not an exclusive recommendation.
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Distributors
Product Focus: Meenakshi Dental Chair – Key Buyer Considerations for 2026
Frequently Asked Questions (FAQs)
| Question | Answer |
|---|---|
| 1. What voltage and electrical specifications are required for the Meenakshi Dental Chair in 2026? | The Meenakshi Dental Chair 2026 Series operates on a standard input voltage of 220–240V AC, 50/60 Hz, with a power consumption of 850W. It supports single-phase power supply and includes an integrated surge protector. A dedicated 10A circuit is recommended for optimal performance. Voltage compatibility varies by regional model; confirm local certification (CE, BIS, or ISO 60601-1) with your distributor prior to installation. |
| 2. Are spare parts for the Meenakshi Dental Chair readily available, and what is the supply chain reliability? | Yes, all critical spare parts—including motors, control panels, upholstery kits, and dental unit attachments—are maintained in regional distribution hubs across India and key international markets. Meenakshi Dental guarantees 10-year availability of essential components post-discontinuation. Distributors receive quarterly inventory updates, and an online spare parts portal (accessible via MeenakshiConnect 2026) enables real-time ordering with 72-hour dispatch for in-stock items. |
| 3. Does the purchase include professional installation, and what does the setup process entail? | Installation is included with all direct and distributor-purchased Meenakshi Dental Chairs in 2026. Certified biomedical engineers conduct on-site setup within 5 business days of delivery. The process includes mechanical assembly, electrical safety testing, hydraulic calibration, integration with dental units (if applicable), and operator training. Clinics must ensure plumbing (for spittoon models) and electrical outlets are pre-routed per the site preparation checklist provided at time of order. |
| 4. What is the warranty coverage for the Meenakshi Dental Chair, and what does it include? | The Meenakshi Dental Chair 2026 model comes with a comprehensive 5-year limited warranty. Coverage includes: hydraulic lift mechanism (5 years), electrical components (3 years), control systems (3 years), and upholstery (1 year, extendable via service contract). Wear-and-tear items (casters, armrest pads) are excluded. Warranty is valid upon registration within 30 days of installation and requires annual preventive maintenance by authorized technicians. |
| 5. How does Meenakshi ensure long-term service support and technical assistance post-warranty? | Meenakshi Dental offers a Post-Warranty Care Program (PWCP-2026) with tiered service plans (Basic, Premium, Platinum). All clinics gain access to 24/7 remote diagnostics via the MeenakshiCare App, which monitors system health and predicts component failure. On-site technical support is available in 200+ cities across India, with a 48-hour response guarantee for registered service partners. Firmware updates and compatibility with emerging dental IoT systems are provided at no additional cost until 2031. |
Need a Quote for Meenakshi Dental Chair?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


