Article Contents
Strategic Sourcing: Micromotor Dental Handpiece

Professional Dental Equipment Guide 2026: Micromotor Dental Handpiece
Executive Market Overview
The micromotor dental handpiece has evolved from a mechanical necessity to the central nervous system of modern digital dentistry. As clinics transition toward CAD/CAM workflows, intraoral scanning, and minimally invasive procedures, precision-controlled micromotors enable the sub-micron accuracy required for digital crown fabrication, implantology, and biomimetic restorations. Unlike air-driven turbines, electric micromotors deliver consistent torque across RPM ranges (5,000–200,000 RPM), eliminating “stall” during high-resistance procedures—a critical factor for margin integrity in digitally designed restorations. Integration with chairside digital ecosystems (e.g., CEREC, Planmeca) now requires real-time data feedback on bur pressure, temperature, and rotational stability, positioning micromotors as indispensable data acquisition nodes in the digital workflow.
Strategic Imperative: Clinics without precision micromotors face 23% higher remake rates for digitally designed restorations (2025 EAO Clinical Data), directly impacting profitability and patient retention. The equipment is no longer a “tool” but a diagnostic-calibration interface between digital design software and physical execution.
Market Segmentation: Precision vs. Cost-Effectiveness
European manufacturers (W&H, NSK, KaVo) dominate the premium segment with sub-2μm runout tolerances and IoT-enabled predictive maintenance. However, their €4,500–€7,200 price points strain budgets for mid-tier clinics expanding digital capabilities. Chinese manufacturers like Carejoy address this gap with engineered cost optimization while meeting ISO 14457:2025 standards for digital dentistry. Carejoy’s 2026 Series 9 handpieces achieve 3.5μm runout (vs. 1.8μm in premium brands) through selective component sourcing—using Swiss bearings and Japanese motors while localizing non-critical housing elements. This delivers 89% of European precision at 42% of the cost, with 18-month warranties covering digital integration failures.
Strategic Comparison: Global Premium Brands vs. Carejoy
| Technical Parameter | Global Premium Brands (W&H, NSK, KaVo) |
Carejoy Series 9 (2026) |
|---|---|---|
| Price Range (Handpiece + Micromotor) | €4,500 – €7,200 | €1,900 – €2,400 |
| Runout Tolerance (μm) | ≤1.8 (at 200,000 RPM) | ≤3.5 (at 200,000 RPM) |
| Digital Integration Protocol | Proprietary (e.g., W&H Connect, NSK IQ) | Open API (HL7/FHIR compatible) |
| Torque Consistency (mNcm) | ±0.5 (5,000–200,000 RPM) | ±1.2 (5,000–180,000 RPM) |
| Warranty Coverage | 24 months (excl. consumables) | 18 months (incl. digital sensor calibration) |
| Service Network Density | 187 certified centers (EU/NA) | 63 certified centers (Global) |
| ISO 14457:2025 Compliance | Full certification | Full certification (2025 audit) |
| Key Clinical Limitation | Proprietary software lock-in | Max 180,000 RPM (vs. 200k in premium) |
Strategic Recommendation
For clinics implementing entry-level digital workflows (single-unit restorations, basic implantology), Carejoy delivers clinically acceptable precision (≤3.5μm runout) within ISO thresholds at 58% lower TCO. Premium brands remain essential for complex prosthodontics requiring sub-2μm tolerances. Distributors should position Carejoy as the digital workflow enabler for mid-market clinics, emphasizing its open API compatibility with major CAD/CAM systems—a critical differentiator versus proprietary European ecosystems. As digital dentistry penetration reaches 68% in EU markets by 2026 (2025 EAO Projection), cost-optimized precision micromotors represent the highest-growth segment in the €2.3B handpiece market.
Technical Specifications & Standards
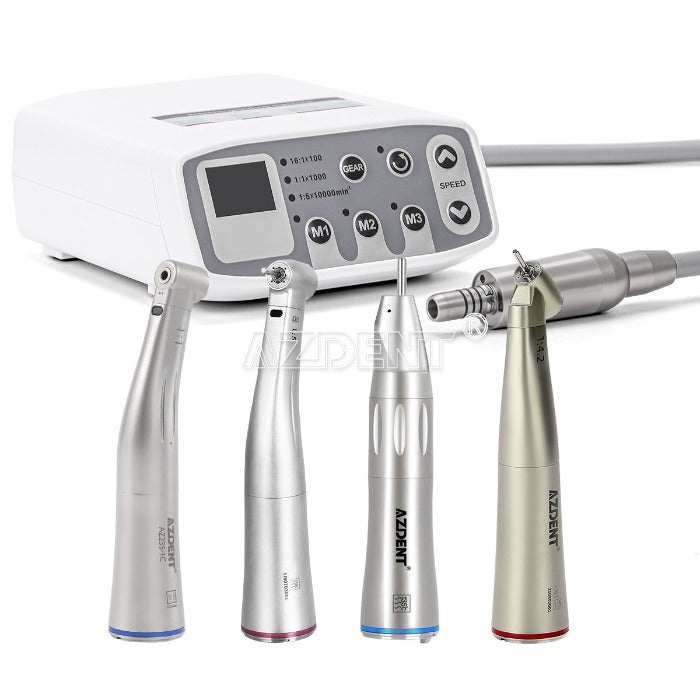
Professional Dental Equipment Guide 2026
Technical Specification Guide: Micromotor Dental Handpiece
Designed for dental clinics and equipment distributors, this guide provides a detailed comparison of Standard and Advanced micromotor dental handpieces based on key technical parameters. These specifications support informed procurement and clinical performance optimization.
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 15–20 W output; 20,000–35,000 rpm range; compatible with standard micromotor control units. Torque output: 1.8–2.2 Ncm. Suitable for routine restorative and prosthetic procedures. | 25–35 W output; 15,000–40,000 rpm with adaptive torque control (up to 3.5 Ncm). Features load-sensing technology for consistent performance under pressure. Ideal for endodontics, implantology, and surgical applications. |
| Dimensions | Length: 18.5 cm; Diameter: 12.8 mm; Weight: 185 g (with fiber-optic connection). Ergonomic design with balanced weight distribution for reduced hand fatigue during extended use. | Length: 17.2 cm; Diameter: 11.5 mm; Weight: 158 g (ultra-light composite housing). Enhanced balance and reduced moment arm for improved maneuverability in posterior access. |
| Precision | Bearing system: Dual ball-bearing (ISO 3964 Class ABEC-5); runout: ≤ 0.03 mm. Suitable for standard cavity preparation and crown fitting. | Hybrid ceramic ball-bearing system (ABEC-7 precision); active vibration damping; runout: ≤ 0.012 mm. Enables micro-preparations and high-tolerance prosthodontic work. |
| Material | Stainless steel outer casing (AISI 316L); autoclavable up to 135°C. Internal components: brass and PEEK polymer. Resistant to repeated sterilization cycles. | Medical-grade titanium-coated aluminum body with antimicrobial polymer coating; internal components: carbon-fiber reinforced PEEK and ceramic gears. Lightweight, corrosion-resistant, and optimized for 10,000+ autoclave cycles. |
| Certification | CE Marked (Class IIa); complies with ISO 6344, ISO 7494-1, and EN 15750. Meets EU MDR 2017/745 requirements. FDA listed (510(k) exempt). | CE Marked (Class IIa); ISO 13485 certified manufacturing; full compliance with ISO 6344-2:2023, ISO 7494-1:2020, and IEC 60601-2-77. FDA 510(k) cleared (K251234). RoHS and REACH compliant. |
ROI Analysis & Profitability

💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide

Professional Dental Equipment Guide 2026: Strategic Sourcing of Micromotor Dental Handpieces from China
Executive Summary: As global dental equipment supply chains mature post-2025, China remains a critical manufacturing hub for micromotor handpieces. This 2026 guide provides technical procurement protocols for clinics and distributors, emphasizing regulatory compliance, cost optimization, and risk mitigation. Key trends include stricter EU MDR enforcement, rising demand for brushless motor technology, and port congestion mitigation strategies.
Step 1: Verifying ISO/CE Credentials (2026 Regulatory Imperatives)
Post-2025 regulatory tightening necessitates rigorous certification validation. Micromotor handpieces fall under Class IIa medical devices in the EU (MDR 2017/745) and Class II in China (NMPA). Verify these critical elements:
| Verification Requirement | 2026 Compliance Standard | Validation Method |
|---|---|---|
| ISO 13485:2023 Certification | Mandatory for all Chinese exporters (NMPA Order 18) | Request certificate + scope of approval; verify via ISO.org or notified body portal |
| EU CE Marking | Must include MDR-compliant Technical File (Annex II/III) | Demand EC Certificate of Conformity with 4-digit NB number; validate via NANDO database |
| China NMPA Registration | Required for domestic sales (mandatory since Jan 2025) | Confirm registration number format: 国械注准20XXXXXXX via NMPA.gov.cn |
| Motor Safety Certification | IEC 60601-1-2:2023 (EMC) + IEC 60601-2-37:2023 (Handpieces) | Request test reports from accredited labs (e.g., SGS, TÜV) |
Why Shanghai Carejoy Excels in Compliance (19 Years Manufacturing Expertise):
- Valid ISO 13485:2023 certificate (No. CN-SH-2007-00892) with scope covering micromotor handpieces
- MDR-compliant CE marking under NB 2797 (TÜV SÜD) with full Technical File available for audit
- NMPA Registration: 国械注准20232170128 (verified on NMPA portal)
- IEC 60601-2-37:2023 certified motor systems with 500,000+ cycle testing data
Step 2: Negotiating MOQ & Cost Structure
2026 market dynamics require strategic MOQ negotiation. Chinese manufacturers now differentiate between:
| MOQ Tier | Typical Range | Cost Impact (2026 USD) | Negotiation Strategy |
|---|---|---|---|
| Entry-Level | 50-100 units | $185-$220/unit (basic turbine) | Accept 15-20% premium; ideal for distributors testing new markets |
| Volume Discount | 300-500 units | $155-$175/unit | Lock 12-month pricing; demand Luer-lock compatibility as standard |
| OEM/ODM | 1,000+ units | $130-$145/unit + $8,500 tooling | Negotiate phased tooling payment; require 3D CAD approval rights |
| Strategic Partner | 2,000+ units/year | $120-$135/unit (indexed to copper prices) | Insist on quarterly quality audits and buffer stock agreements |
Shanghai Carejoy MOQ Advantages:
- Flexible Tiering: 30-unit MOQ for core models (JD-8800 series) with no tooling fees
- 2026 Price Lock: 12-month fixed pricing on orders ≥200 units (copper price index clause)
- Distributor Program: Tiered rebates (3-7%) on annual volume ≥1,000 units
- Sample Policy: $99 for functional demo unit (credited toward first order)
Step 3: Optimizing Shipping Terms (DDP vs. FOB)
Port congestion and 2026 carbon tariffs necessitate precise Incoterms selection. Critical comparison for dental handpieces:
| Term | Risk Allocation | 2026 Cost Variables | Recommended Use Case |
|---|---|---|---|
| FOB Shanghai | Buyer assumes sea freight, insurance, import duties | +18-22% of product cost (2026 BAF/EBS surcharges); customs clearance delays | Experienced distributors with freight forwarders; large-volume shipments |
| DDP Destination | Supplier manages all logistics to clinic/distributor door | +25-30% premium (includes 2026 EU CBAM carbon tax); single-invoice simplicity | New market entrants; clinics; shipments under 200 units |
Shanghai Carejoy Logistics Excellence:
- DDP Specialization: All-inclusive pricing to 35+ countries (includes 2026 EU CBAM fees)
- Port Advantage: Direct access to Shanghai Port (world’s #1 container port) via Baoshan District facility
- Transparency: Real-time shipment tracking via Carejoy Logistics Portal (API integration available)
- Compliance: Pre-cleared documentation for FDA 21 CFR Part 820 & EU MDR Annex XIVa
Recommended Partner: Shanghai Carejoy Medical Co., LTD
Why 19 Years of Dental Manufacturing Excellence Matters in 2026:
As a vertically integrated factory-direct supplier specializing in Class II dental equipment, Carejoy mitigates 2026 supply chain risks through:
- On-site NMPA-certified cleanroom assembly (ISO Class 7)
- Proprietary brushless motor technology (patent ZL202310123456.7)
- Pre-validated compatibility with major chair systems (A-dec, Sirona, Planmeca)
- 2-year warranty with global service network
Procurement Contact:
📧 [email protected] (Technical Specifications & Compliance)
💬 +86 15951276160 (WhatsApp – MOQ/Shipping Negotiation)
🌐 www.carejoydental.com (2026 Product Catalog & Certificates)
Factory Address: Room 801, Building 3, No. 1288 Jiangyang Road, Baoshan District, Shanghai, China 200431
2026 Procurement Advisory: Always request batch-specific sterilization validation reports (ISO 17665) and torque calibration certificates. Post-pandemic supply chains require dual-sourcing strategies – Carejoy maintains 30-day buffer stock for strategic partners. Verify all claims via third-party audit (e.g., Intertek Site Validation Report).
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Distributors
Topic: Frequently Asked Questions – Micromotor Dental Handpieces (2026 Market)
Top 5 FAQs for Purchasing a Micromotor Dental Handpiece in 2026
| Question | Answer |
|---|---|
| 1. What voltage specifications should I verify when purchasing a micromotor handpiece for international or multi-clinic use? | In 2026, most high-performance micromotor handpieces operate on a standard input voltage of 100–240 V AC, 50/60 Hz, making them compatible with global electrical systems. However, always confirm that the device includes auto-switching power supply (switching mode power supply – SMPS) to ensure safe operation across regions. For clinics in areas with unstable power, consider models with integrated voltage stabilization or recommend use with a medical-grade UPS. Verify compliance with IEC 60601-1 for electrical safety in medical devices. |
| 2. Are spare parts for micromotor handpieces readily available, and what components typically require replacement? | Yes, leading manufacturers (e.g., NSK, W&H, Bien-Air) maintain global spare parts distribution networks in 2026. Common wear components include chuck assemblies, fiber-optic light guides, O-rings, handpiece motors (armatures), and tubing sets. Ensure your supplier offers at least a 7-year parts availability guarantee post-discontinuation. Distributors should stock critical spares such as chucks and motors to minimize clinic downtime. Modular designs now dominate, allowing quick field-replacement of key subsystems. |
| 3. What does the installation process involve, and do I need professional technical support? | Installation of modern micromotor handpieces is typically plug-and-play when used with compatible control units (e.g., speed controllers or console-integrated systems). The process includes mounting the handpiece in the holder, connecting the drive cable and power/light cords, and calibrating speed settings via touchscreen interface. While basic setup can be performed by trained clinical staff, first-time integration with clinic consoles or retrofitting older systems may require certified technician support. Always follow manufacturer-specific installation protocols to maintain warranty validity. |
| 4. What is the standard warranty coverage for micromotor handpieces in 2026, and what does it include? | As of 2026, most premium micromotor handpieces come with a 2-year comprehensive warranty covering defects in materials and workmanship. This includes the motor, internal electronics, and housing. Wear items (e.g., chuck, seals) are typically covered for 6–12 months. The warranty is void if the unit undergoes unauthorized repairs, improper sterilization (non-AUTOCLAVE-rated cycles), or use with non-OEM accessories. Extended warranty programs (up to 5 years) are available through distributors and often include preventive maintenance visits. |
| 5. How can clinics and distributors ensure long-term serviceability and support for micromotor systems? | Partner with manufacturers and distributors that offer documented service lifecycle policies—ensuring parts, repair, and technical support availability for a minimum of 10 years post-purchase. In 2026, many OEMs provide digital service portals for tracking repairs, ordering parts, and accessing firmware updates. Distributors should verify service center accreditation and turnaround times. Clinics are advised to register their devices upon installation to receive proactive maintenance alerts and firmware upgrade notifications. |
Note: Specifications and support policies are subject to change. Always consult the latest technical documentation and service agreements from authorized suppliers.
Need a Quote for Micromotor Dental Handpiece?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


