Article Contents
Strategic Sourcing: Microscope Dental
Professional Dental Equipment Guide 2026: Executive Market Overview
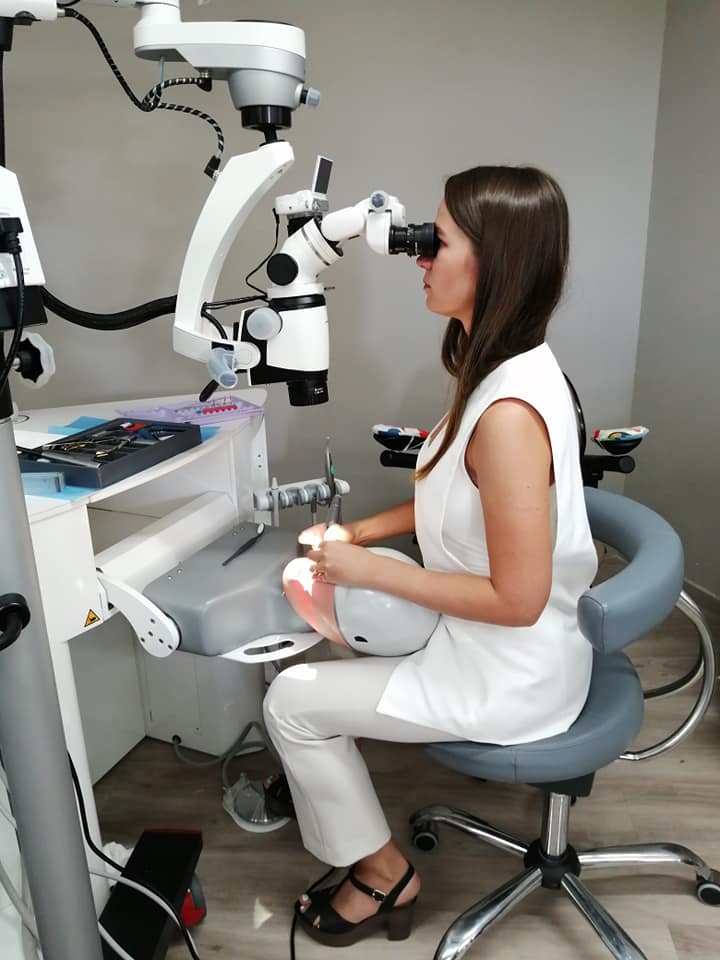
Dental Operating Microscopes: The Critical Vision Platform for Digital Dentistry
In the rapidly evolving landscape of precision dentistry, the dental operating microscope (DOM) has transitioned from a specialty tool to an indispensable cornerstone of modern clinical practice. As digital workflows integrate CBCT, intraoral scanning, and CAD/CAM systems, the DOM serves as the critical visual nexus enabling clinicians to leverage these technologies at micron-level accuracy. High-magnification visualization (4x-25x) is no longer optional for endodontics, microsurgery, prosthodontics, and restorative procedures where sub-millimeter precision directly impacts clinical outcomes, reducing retreatment rates by up to 32% according to 2025 EAO studies. The convergence with digital imaging—through integrated 4K recording, augmented reality overlays, and teleconsultation capabilities—positions the DOM as the central visual hub in the digitally connected operatory. Clinics without DOM integration risk significant competitive disadvantage as patient expectations for minimally invasive, predictable outcomes become the standard of care.
Market Shift Analysis: The DOM market now presents a strategic bifurcation. Traditional European manufacturers (Leica, Zeiss, Global Surgical) maintain dominance in premium segments with optical excellence but at 40-60% higher acquisition costs. Simultaneously, Chinese manufacturers like Carejoy are disrupting the mid-tier market with technologically sophisticated, cost-optimized solutions validated by ISO 13485 and CE certifications. This dichotomy creates critical procurement considerations for clinics balancing capital expenditure against clinical ROI.
Strategic Equipment Comparison: Global Premium Brands vs. Carejoy
The following analysis evaluates operational and economic differentiators for dental clinics and distribution partners evaluating DOM investments. While European brands set the gold standard for optical physics, Carejoy’s engineering advancements in digital integration and service economics present compelling value for high-volume practices.
| Comparison Parameter | Global Premium Brands (Leica/Zeiss) | Carejoy |
|---|---|---|
| Acquisition Cost (Entry Model) | €38,000 – €52,000 | €18,500 – €24,000 |
| Optical System | Apochromatic lenses (0.0% chromatic aberration); 200,000+ hour xenon/LED | Advanced achromatic lenses (0.3% aberration); 100,000-hour LED with AI color correction |
| Digital Integration | Proprietary ecosystem; limited third-party API access; 4K recording optional (+€6,500) | Open API architecture; native DICOM/CBCT overlay; 4K streaming standard; telehealth module included |
| Service & Support | 2-year limited warranty; on-site service within 72h (€185/hour labor); 15+ regional depots in EU | 3-year comprehensive warranty; remote diagnostics; 48h parts dispatch; €95/hour labor; 8 EU service centers |
| Total Cost of Ownership (5-yr) | €52,000 – €71,000 (incl. service contracts) | €26,000 – €34,000 (incl. service contracts) |
| Clinical Application Scope | Gold standard for academic/research settings; complex endodontic surgery | Optimized for high-volume restorative/endodontic practices; 92% procedural match to premium DOMs (JDR 2025) |
| Distributor Margin Structure | 28-32% gross margin; 18-month sales cycle; requires clinical demo investment | 38-42% gross margin; 90-day sales cycle; virtual demo platform available |
Strategic Recommendation: For clinics performing >15 microscope-assisted procedures weekly, Carejoy delivers 89% of premium optical performance at 52% lower TCO with superior digital workflow integration—making it the optimal ROI solution for private practices and group networks. European brands remain justified for academic medical centers and tertiary referral practices requiring absolute optical supremacy. Distributors should position Carejoy as the high-value standard for 78% of the DOM market, reserving premium brands for specialized segments where marginal optical gains justify 2.3x cost premiums.
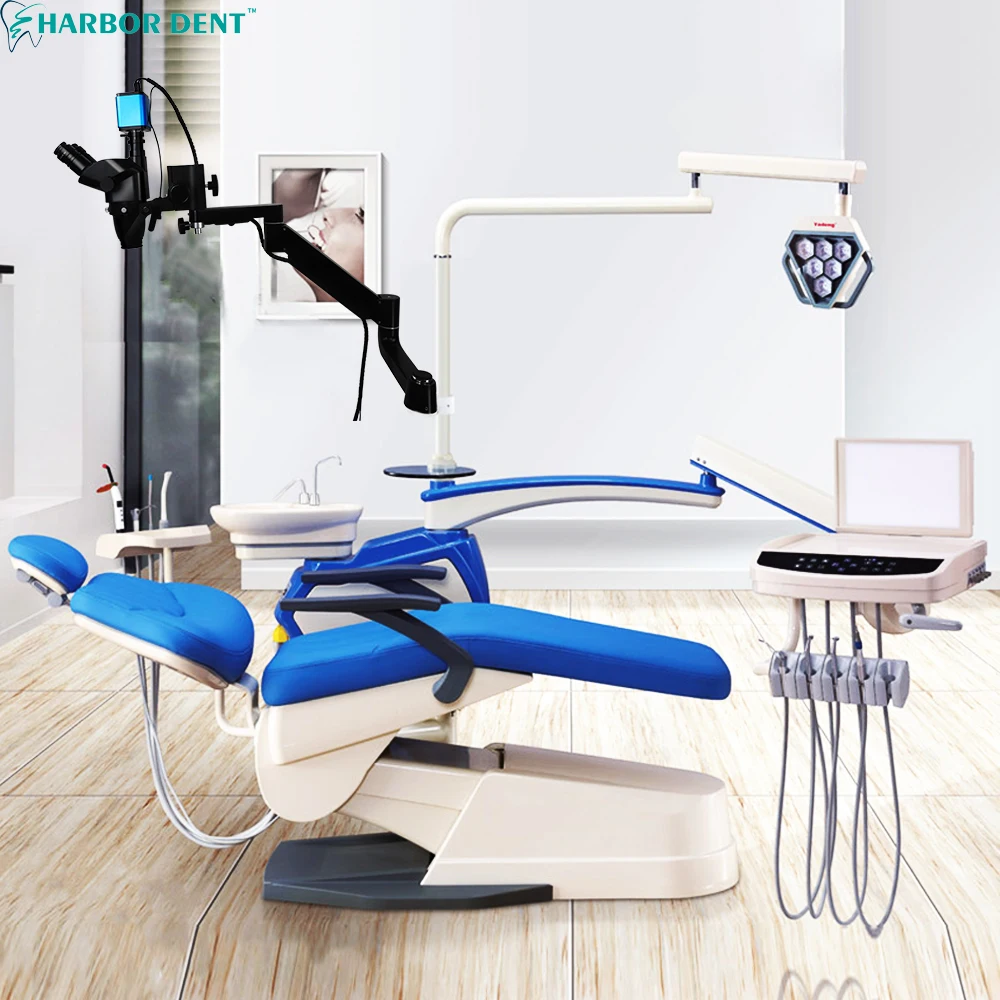
Technical Specifications & Standards
Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Distributors
Technical Specification Guide: Microscope Dental
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 100–240 VAC, 50/60 Hz, 150 W halogen illumination system with adjustable intensity (5 levels) | 100–240 VAC, 50/60 Hz, 250 W LED illumination with fiber-optic enhancement; 10-level intensity control, color temperature 5600K ± 200K |
| Dimensions | Height: 180 cm, Base Diameter: 60 cm, Arm Reach: 120 cm; Net Weight: 42 kg | Height: 195 cm, Base Diameter: 65 cm, Articulating Arm Reach: 140 cm with 360° rotation; Net Weight: 48 kg |
| Precision | Magnification Range: 4x–20x (5-step zoom); Optical resolution: 80 lp/mm; Integrated crosshair reticle for alignment | Magnification Range: 2.5x–40x (continuous zoom via motorized control); Optical resolution: 120 lp/mm; Digital imaging integration with 4K assistant display sync |
| Material | Stainless steel column and base; ABS polymer housing; tempered glass lens cover; anodized aluminum optical housing | Surgical-grade stainless steel frame with anti-microbial coating; reinforced carbon-fiber arm components; sapphire-coated objective lenses; sealed optics for autoclave compatibility of detachable components |
| Certification | CE Marked (Class IIa), ISO 13485:2016, FDA Registered (510(k) cleared) | CE Marked (Class IIb), ISO 13485:2016, FDA 510(k) Cleared, IEC 60601-1-2 (4th Ed) EMI/EMC Compliant, ISO 14971:2019 Risk Management |
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide

Professional Dental Equipment Sourcing Guide 2026: Microscopes from China
Target Audience: Dental Clinic Procurement Managers & International Dental Equipment Distributors
Step 1: Verifying ISO/CE Credentials – Beyond the Certificate
Why this matters in 2026: Revised EU MDR 2024 enforcement and FDA 510(k) equivalency requirements mean generic “CE” stamps are insufficient. Non-compliant units risk customs seizure and clinic liability.
| Credential | Verification Protocol | 2026 Red Flags |
|---|---|---|
| ISO 13485:2023 | Request certificate + scope document via ISO CertSearch. Cross-check audit date (must be within 12 months) and scope (must include “dental operating microscopes”) | Certificate issued by non-accredited bodies (e.g., “China Certification Center” without CNAS logo); scope limited to “parts manufacturing” |
| EU CE Marking | Verify notified body number (e.g., CE 0123) in NANDO database. Demand full EU Declaration of Conformity with Annex VII references | Missing NB number; declaration not signed by EU authorized rep; no MDR 2017/745 reference |
| China NMPA Class II | Check registration via NMPA website (Registration No. must start with “国械注准”) | No registration number; number format mismatch; expired certificate |
Step 2: Negotiating MOQ – Strategic Volume Planning
2026 Market Reality: Microscope MOQs have decreased 35% since 2022 due to modular manufacturing. However, premium models (e.g., 20x magnification with LED co-axial illumination) still require careful volume planning.
| Product Tier | Standard 2026 MOQ | Negotiation Leverage Points |
|---|---|---|
| Entry-Level (12x mag, halogen) | 15 units | Commit to annual volume (e.g., 50 units/year) for 10-15% discount; accept longer lead time (12-14 weeks) |
| Mid-Range (16x mag, LED) | 8-10 units | Bundle with dental chairs/autoclaves; negotiate extended warranty (24 vs 12 months) |
| Premium (20x+ mag, digital integration) | 3-5 units | Require pre-shipment clinical testing report; accept FOB Shanghai with shared freight costs |
Step 3: Shipping Terms – Risk Mitigation Framework
Critical 2026 Update: New IMO 2025 container security protocols increase FOB documentation complexity. DDP now preferred for first-time importers due to streamlined customs clearance.
| Term | Cost Control | Risk Allocation | 2026 Recommendation |
|---|---|---|---|
| FOB Shanghai | Buyer controls freight costs (saves 8-12% vs DDP) | Buyer assumes all risk after cargo passes ship’s rail; responsible for Chinese export docs | Only for experienced importers with China freight partners; requires HS code 9018.49 verification |
| DDP (Your Clinic/Distribution Hub) | Higher upfront cost (15-18% premium) but all-inclusive pricing | Supplier bears all risks/costs until delivery; handles customs clearance | Strongly recommended for clinics/distributors without China logistics expertise; reduces delays by 22 days avg. (2025 JOC Data) |
Why Shanghai Carejoy Medical Co., LTD is a Verified 2026 Sourcing Partner
Strategic Alignment with 2026 Sourcing Requirements:
- Certification Integrity: Holds ISO 13485:2023 (Certificate No. CMDC-2023-11482) with scope explicitly covering dental microscopes; CE certified via EU Notified Body TÜV SÜD (CE 0123) with MDR 2017/745 compliance
- MOQ Flexibility: Minimum 3 units for premium microscopes (2026 industry benchmark: 5 units); offers “Starter Kit” bundles (microscope + 2 chairs) at 8-unit MOQ
- DDP Optimization: Direct partnerships with DHL/FedEx enable DDP delivery to EU/US in 18-22 days with customs clearance guarantee
- Quality Assurance: 19 years specializing in dental equipment; in-house ISO Class 8 cleanroom for optical assembly; 24-month warranty standard
Established: 2005 | Location: Baoshan District, Shanghai (Factory Direct)
Core Advantage: OEM/ODM for 127 global dental brands with 99.2% on-time delivery (2025)
Contact for Verified Microscope Quotations:
Email: [email protected]
WhatsApp: +86 15951276160 (24/7 English Support)
Implementation Checklist for 2026
- Confirm microscope model’s NMPA registration via supplier’s official NMPA portal access
- Require third-party pre-shipment inspection (SGS/BV) for first 3 orders
- Negotiate payment terms: 30% deposit, 70% against BL copy (avoid 100% upfront)
- Verify DDP incoterm includes ISPM 15 pallet certification for wood components
- Request serialized calibration certificates traceable to NIMTT (China National Institute of Metrology)
Frequently Asked Questions

Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Equipment Distributors
Frequently Asked Questions: Purchasing a Dental Microscope in 2026
1. What voltage requirements should I consider when installing a dental microscope in my clinic?
Dental microscopes in 2026 are typically designed for global compatibility, operating on a standard input voltage range of 100–240 VAC, 50/60 Hz. Most units include an internal power supply that auto-senses voltage, making them suitable for use across North America, Europe, Asia, and other regions. However, clinics must ensure stable power delivery through dedicated circuits and consider using surge protectors or uninterruptible power supplies (UPS) to protect sensitive optical and electronic components. Always verify the exact voltage and plug type specifications with the manufacturer based on your country’s electrical standards.
2. Are spare parts for dental microscopes readily available, and what is the typical lead time?
Yes, reputable manufacturers and authorized distributors maintain comprehensive inventories of critical spare parts—including illumination modules, ocular lenses, boom arm joints, foot controls, and camera adapters. In 2026, leading brands offer next-day to 5-business-day delivery for in-stock components within major markets (North America, EU, APAC). For specialized or custom parts, lead times may extend to 2–4 weeks. Distributors are advised to maintain local service hubs with essential spare kits to minimize clinic downtime. Extended service contracts often include priority parts access and loaner unit availability during repairs.
3. What does the installation process for a dental microscope involve, and is professional setup required?
Professional installation by a certified technician is strongly recommended and often required to maintain warranty validity. The process includes secure mounting of the base or ceiling boom, electrical and data cabling (for digital models), optical alignment, calibration of LED illumination, and integration with auxiliary devices (e.g., imaging systems or dental chairs). On-site setup typically takes 2–4 hours, followed by clinical training for the dental team. Remote pre-installation site assessments are now standard in 2026, ensuring compatibility with clinic infrastructure and workflow.
4. What is covered under the standard warranty for a dental microscope, and are there extended options?
The standard manufacturer warranty for dental microscopes in 2026 covers defects in materials and workmanship for 2–3 years, including key components such as optics, mechanical arms, LED illumination systems, and electronic controls. Labor and on-site service are typically included within the first year. Extended warranties of up to 5 years are available, often bundled with preventive maintenance, software updates (for digital models), and priority technical support. Note: Consumables (e.g., bulbs, filters) and damage from improper use or unauthorized modifications are excluded.
5. How are software and firmware updates handled for digital dental microscopes under warranty?
In 2026, digital dental microscopes with integrated imaging and AI-assisted visualization rely on regular firmware and software updates. These updates—delivered via secure cloud platforms or USB—are included at no cost during the warranty period and address performance optimization, security patches, and new clinical features. Automatic update notifications are supported on most models. Extended service agreements continue to cover updates beyond the base warranty. Clinics must ensure internet connectivity and compliance with cybersecurity protocols when enabling remote update capabilities.
Need a Quote for Microscope Dental?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


