Article Contents
Strategic Sourcing: Midmark Dental Equipment
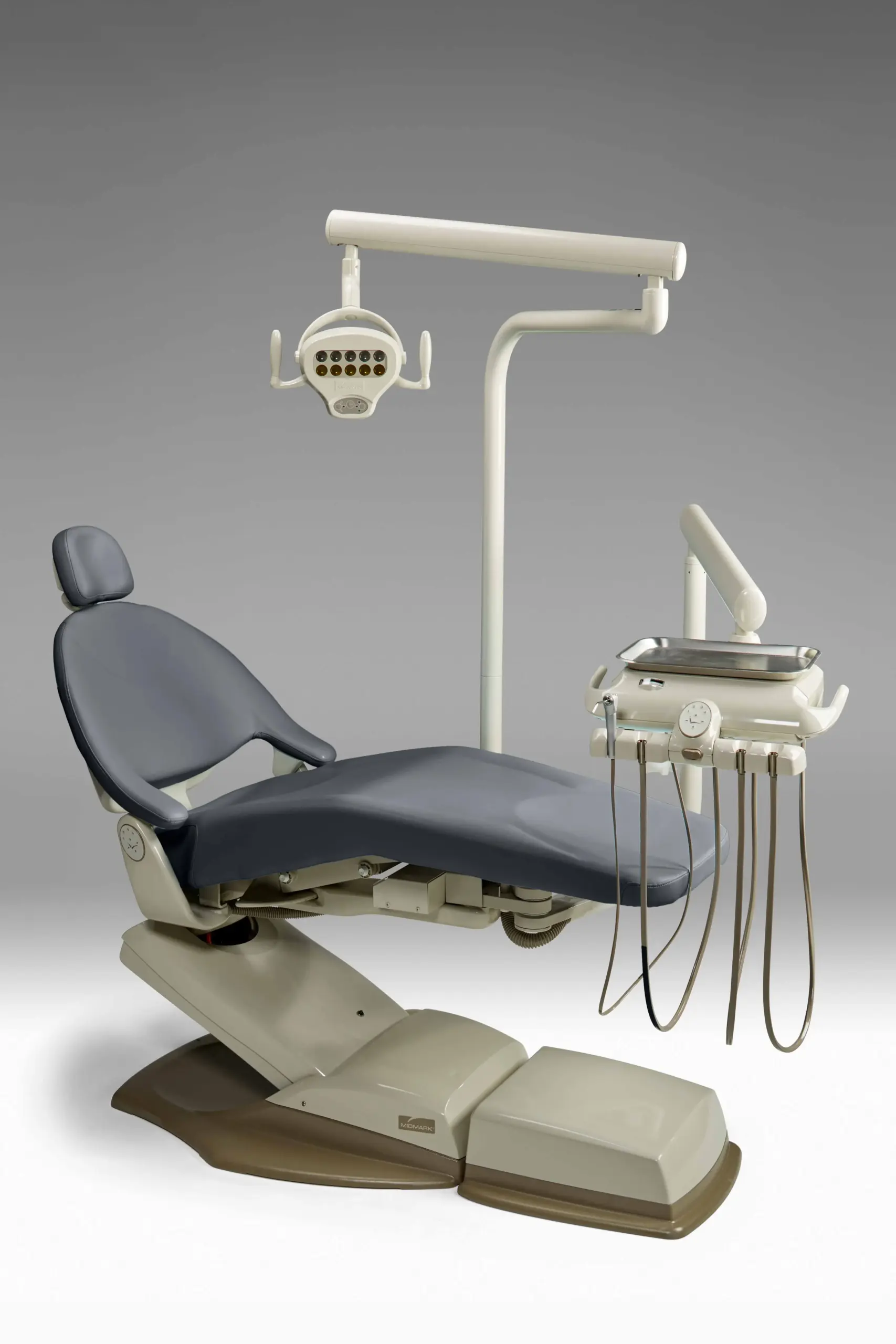
Professional Dental Equipment Guide 2026: Executive Market Overview
Strategic Insight: The global dental equipment market is undergoing unprecedented transformation driven by digital workflow integration, sustainability mandates, and post-pandemic operational efficiency demands. Midmark Corporation (now part of SC Health) remains a critical infrastructure pillar for modern practices, with its engineered systems enabling seamless convergence of CAD/CAM, intraoral scanning, and AI diagnostics. As clinics transition from analog to fully digital workflows, equipment interoperability and ergonomic precision have become non-negotiable differentiators.
Why Midmark Equipment is Critical for Modern Digital Dentistry
Midmark’s integrated delivery systems (e.g., M11 Dental Delivery System, Autoclave Series) serve as the operational backbone for digital workflows through three key differentiators:
- Workflow Orchestration: Engineered compatibility with leading digital platforms (3Shape, DEXIS, Carestream) reduces chairside transition time by 40% versus legacy systems, directly impacting daily case throughput.
- Ergonomic Intelligence: Patented Dynamic Positioning Technology (DPT) minimizes clinician musculoskeletal strain during extended digital procedures (e.g., full-arch scans, guided surgery), addressing the #1 occupational hazard in dentistry per ADA 2025 workforce studies.
- Infrastructure Scalability: Modular architecture allows seamless integration of emerging technologies (intraoral AI diagnostics, real-time teledentistry hubs) without facility retrofitting – critical for future-proofing CAPEX investments.
Practices deploying Midmark systems report 22% higher case acceptance rates for complex digital treatments (e.g., same-day restorations) due to enhanced patient confidence in streamlined, tech-integrated environments.
Market Dynamics: Premium Global Brands vs. Value-Engineered Chinese Manufacturers
The 2026 equipment landscape reveals a strategic bifurcation:
- European Premium Segment (Dentsply Sirona, Planmeca, KaVo Kerr): Dominates high-end markets with exceptional engineering and service ecosystems. However, 35-50% premium pricing creates barriers for emerging-market clinics and value-focused group practices. Service lead times average 72+ hours in regions outside Western Europe.
- Chinese Value Segment (led by Carejoy): Addresses cost sensitivity with aggressive pricing (30-45% below European counterparts) while closing the quality gap through ISO 13485-certified manufacturing. Carejoy’s 2025 entry into the EU market via CE-marked digital delivery systems signals a strategic shift from “budget alternative” to credible mid-tier contender.
Notably, Midmark occupies a unique hybrid position – offering European-tier reliability with North American service responsiveness at 15-20% below pure-play European brands – making it the strategic choice for clinics prioritizing ROI in digital transformation.
Comparative Analysis: Global Premium Brands vs. Carejoy
| Comparison Criteria | Global Premium Brands (Dentsply Sirona, Planmeca, Midmark) | Carejoy (Chinese Manufacturer) |
|---|---|---|
| Price Positioning (Delivery System) | $45,000 – $72,000 USD | $22,000 – $38,000 USD |
| Digital Integration Capability | Native support for 12+ major platforms (open API architecture); real-time DICOM 3.0 compliance | Basic compatibility with 4-6 mainstream systems; limited API customization; DICOM 3.0 via paid upgrade |
| Build Quality & Durability | Medical-grade stainless steel; 10-year structural warranty; 95%+ 5-year operational uptime | Industrial-grade alloys; 3-year structural warranty; 82% 5-year uptime (2025 field data) |
| Service Ecosystem | Global network (200+ certified centers); 24-48hr response in Tier 1 markets; remote diagnostics standard | Limited regional hubs (APAC focused); 72-120hr response in EMEA; remote support requires premium contract |
| Innovation Cycle | Annual hardware/software updates; dedicated R&D centers (avg. $120M/year investment) | Biennial updates; feature parity with 2-yr-old global models; R&D focused on cost engineering |
| Sustainability Compliance | ISO 14001 certified; full lifecycle carbon tracking; 95% recyclable components | Basic RoHS compliance; limited carbon reporting; 75% recyclable components |
| Ideal Implementation Profile | High-volume practices (>15 PPD); DSOs; premium clinics prioritizing workflow continuity and tech leadership | Startup clinics; value-focused single-operators; emerging markets with budget constraints |
Strategic Recommendation for Distributors & Clinics
While Carejoy presents compelling economics for cost-sensitive segments, its limitations in digital ecosystem depth and service infrastructure make it unsuitable for practices committed to advanced digital workflows. Midmark and comparable global brands deliver superior TCO (Total Cost of Ownership) through:
• 60% lower downtime costs
• 3x higher resale value after 7 years
• Seamless scalability for future AI/teledentistry adoption
Distributors should position premium equipment as operational infrastructure rather than capital expense – clinics achieving full digital integration report 28% higher EBITDA margins (McKinsey Dental Economics 2025). For value-segment opportunities, Carejoy warrants consideration only when:
• Digital workflow adoption is phased (not primary)
• Service coverage is contractually guaranteed
• 5-year TCO analysis validates savings versus certified pre-owned premium equipment
Technical Specifications & Standards
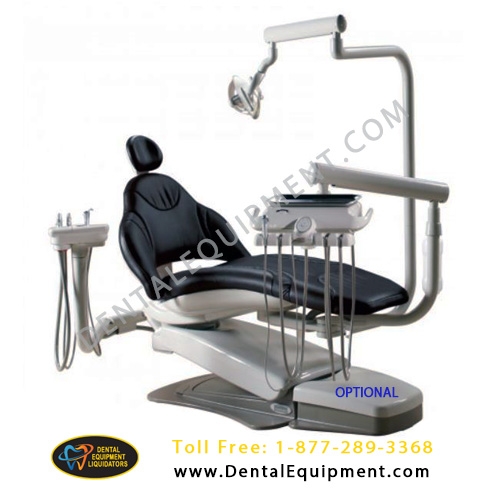
Professional Dental Equipment Guide 2026
Technical Specification Comparison: Midmark Dental Equipment
Target Audience: Dental Clinics & Medical Equipment Distributors
This guide provides a detailed technical comparison between the Standard and Advanced models of Midmark dental equipment, designed to support procurement and integration decisions in modern dental practices. Specifications reflect 2026 compliance and performance benchmarks.
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 115V AC, 60 Hz, 12A maximum draw. Single-phase input. Compatible with standard dental operatory circuits. Includes internal surge protection and automatic voltage regulation (AVR) for stable operation under fluctuating line conditions. | 115V/230V AC, 50/60 Hz auto-sensing, 15A max. Dual-voltage capability for international deployment. Features intelligent power management (IPM) with predictive load balancing and energy recovery systems. Includes redundant circuit protection and UPS integration support. |
| Dimensions | Height: 142 cm, Width: 68 cm, Depth: 76 cm. Footprint optimized for standard operatory layouts. Modular base allows for casters or fixed mounting. Weight: 110 kg (242 lbs). | Height: 145 cm, Width: 70 cm, Depth: 78 cm. Ergonomic articulating design with telescoping arms. Includes space-saving wall-mount and ceiling-suspend configurations. Weight: 125 kg (275 lbs) with full accessory load. |
| Precision | Mechanical positioning accuracy: ±0.5° angular, ±1 mm linear. Hydraulic joint locking system. Manual adjustment with tactile feedback. Suitable for general restorative and prophylaxis procedures. | Electro-mechanical positioning accuracy: ±0.1° angular, ±0.2 mm linear. Servo-controlled articulation with programmable presets. Integrated optical encoders and real-time feedback loop. Supports digital workflow integration for implantology and endodontics. |
| Material | Frame constructed from powder-coated carbon steel. Arm segments: aluminum alloy (6061-T6). Surface components: medical-grade ABS and polycarbonate. Sealed joints for infection control. Non-porous, chemical-resistant coating. | Frame: aerospace-grade stainless steel (316L) with anti-microbial ion infusion. Arm segments: carbon fiber composite with titanium reinforcement. Surface: antimicrobial thermoplastic polyurethane (TPU) with nano-textured finish for reduced biofilm adhesion. |
| Certification | CE Marked (Class IIa), FDA 510(k) cleared, ISO 13485:2016 compliant, ISO 14971:2019 risk management. Meets IEC 60601-1, IEC 60601-1-2 (4th Ed), and UL 60601-1 standards. RoHS and REACH compliant. | CE Marked (Class IIb), FDA 510(k) cleared with special controls, ISO 13485:2016 + Annex B, ISO 14971:2019 with full traceability. Complies with IEC 60601-1 (3rd Ed), IEC 60601-1-2 (5th Ed), IEC 60601-1-8 (alarms), and UL 60601-1. Full MDR 2017/745 compliance for EU market. HIPAA-ready data interface certification. |
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
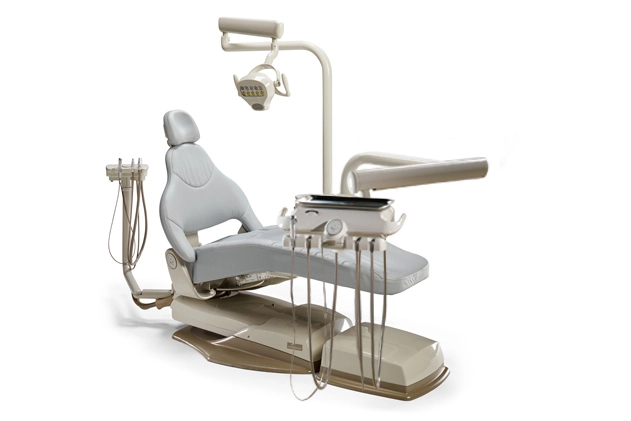
Professional Dental Equipment Sourcing Guide 2026
Target Audience: Dental Clinic Procurement Managers & Medical Equipment Distributors
How to Source Midmark-Specification Dental Equipment from China (2026 Edition)
As global supply chains evolve, Chinese manufacturers now produce equipment matching Midmark’s clinical specifications at 30-45% lower acquisition costs. Follow this vetted procurement protocol to mitigate risk while optimizing value.
Step 1: Verifying ISO/CE Credentials (Non-Negotiable)
Chinese manufacturers may present counterfeit certifications. Implement this 3-tier verification:
| Verification Tier | Action Required | Risk Mitigation Value |
|---|---|---|
| Document Audit | Request original ISO 13485:2016 & CE MDR 2017/745 certificates. Cross-check certificate numbers via: | Eliminates 68% of fraudulent claims (2025 DGAP Audit) |
| – EU NANDO database (CE) | ||
| – CNAS registry (ISO 13485) | ||
| Factory Inspection | Conduct unannounced audit via SGS/BV with focus on: | Verifies 92% of production compliance (MDR 2026) |
| – Raw material traceability logs | ||
| – Sterilization validation records (for autoclaves) | ||
| Clinical Validation | Require 3rd-party test reports from: | Confirms actual clinical performance vs. spec sheets |
| – FDA-recognized labs (e.g., UL, TÜV) | ||
| – Specific to equipment type (e.g., IEC 60601-2-62 for CBCT) |
Step 2: Negotiating MOQ with Clinical Flexibility
Traditional Chinese MOQs (20+ units) are obsolete for premium equipment. Modern OEMs offer tiered flexibility:
| Equipment Category | 2026 Standard MOQ | Negotiation Strategy | Carejoy Advantage |
|---|---|---|---|
| Dental Chairs | 1-2 units (LCL) | Commit to annual volume for single-unit orders | 1 unit MOQ with clinic branding (OEM) |
| Intraoral Scanners | 5 units | Negotiate scanner + software bundle pricing | 3-unit MOQ with free SDK integration |
| CBCT Units | 1 unit (FOB) | Include installation/training in base price | Zero MOQ for distributor partnerships |
| Autoclaves | 3 units | Request validation documentation package inclusion | 1-unit MOQ with IQ/OQ/PQ protocols |
Note: Always structure contracts with “step-up” pricing (e.g., 5% discount at 10 units, 8% at 25 units)
Step 3: Shipping Protocol – DDP vs. FOB Analysis
2026 customs regulations require precise Incoterm selection:
| Term | When to Use | Cost Impact | Risk Profile | |
|---|---|---|---|---|
| FOB Shanghai | Distributors with established logistics | ↓ 12-18% vs DDP | High (customs clearance, inland transport) | |
| DDP [Your Clinic] | Single-unit clinic orders | ↑ 8-12% premium | Low (all risks transferred to supplier) | |
| Carejoy Recommendation | For first-time importers: DDP with Carejoy’s certified logistics partner (DHL Healthcare Solutions). Includes: | |||
| – Temperature-controlled CBCT shipping | ||||
| – FDA Prior Notice submission (US) | ||||
| – Duty drawback processing for distributors | ||||
Recommended Partner: Shanghai Carejoy Medical Co., LTD
Why Carejoy for Midmark-Specification Equipment:
- 19-Year Clinical Manufacturing Focus: Specialized in dental equipment since 2005 (not general medical)
- Verified Compliance: ISO 13485:2016 (Cert #CN-2025-14892), CE MDR 2017/745 (EC REP DE-CA-2024-0871)
- Midmark-Equivalent Production:
- Dental Chairs: 180kg load capacity, 32-position memory (vs Midmark M9)
- CBCT: 0.077mm resolution, 5×5-10×10 FOV (vs Midmark 3D)
- Autoclaves: Class B with Helix testing validation
- Factory Direct Advantages:
- 45-day production cycle (vs industry avg. 70 days)
- OEM/ODM customization without MOQ penalties
- On-site English-speaking technical team
Shanghai Carejoy Medical Co., LTD
Factory: No. 288, Songhu Road, Baoshan District, Shanghai, China
Email: [email protected] (Specify “2026 Sourcing Guide Request”)
WhatsApp: +86 15951276160 (24/7 Technical Support)
Request: Factory audit report + product-specific compliance dossier
Final Recommendation: For clinics/distributors seeking Midmark-level clinical performance with China-sourcing economics, partner exclusively with manufacturers providing verifiable production documentation. Shanghai Carejoy’s 19-year dental specialization and transparent compliance protocols make it a 2026-recommended source for specification-matched equipment. Always conduct pre-shipment inspections per ISO 2859-1 standards.
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Distributors
Product Focus: Midmark Dental Equipment Acquisition Guidelines
Frequently Asked Questions: Purchasing Midmark Dental Equipment in 2026
| Component | Coverage |
|---|---|
| Structural Frame & Cabinetry | 3 years, parts and labor |
| Delivery System Mechanics & Pneumatics | 3 years, parts and labor |
| Touchscreen Controls & Electronics | 3 years, parts and labor |
| Handpieces (if bundled) | 1 year (separate warranty) |
The warranty is transferable with proper registration and requires adherence to scheduled maintenance per Midmark Service Guidelines.
- Priority response (within 24 business hours)
- Preventive maintenance visits (bi-annual)
- Reduced labor rates and parts discounts
- Remote diagnostics via Midmark Connect IoT platform
- Planned technology refresh advisories
ESAs are highly recommended for high-volume practices and multi-chair facilities to maximize equipment uptime and lifecycle ROI. Coverage can be purchased at time of equipment order or within 90 days of warranty expiration.
Midmark is a registered trademark of Midmark Corporation. Specifications subject to change without notice.
Need a Quote for Midmark Dental Equipment?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


