Article Contents
Strategic Sourcing: Midmark Dental Machine
Professional Dental Equipment Guide 2026: Executive Market Overview
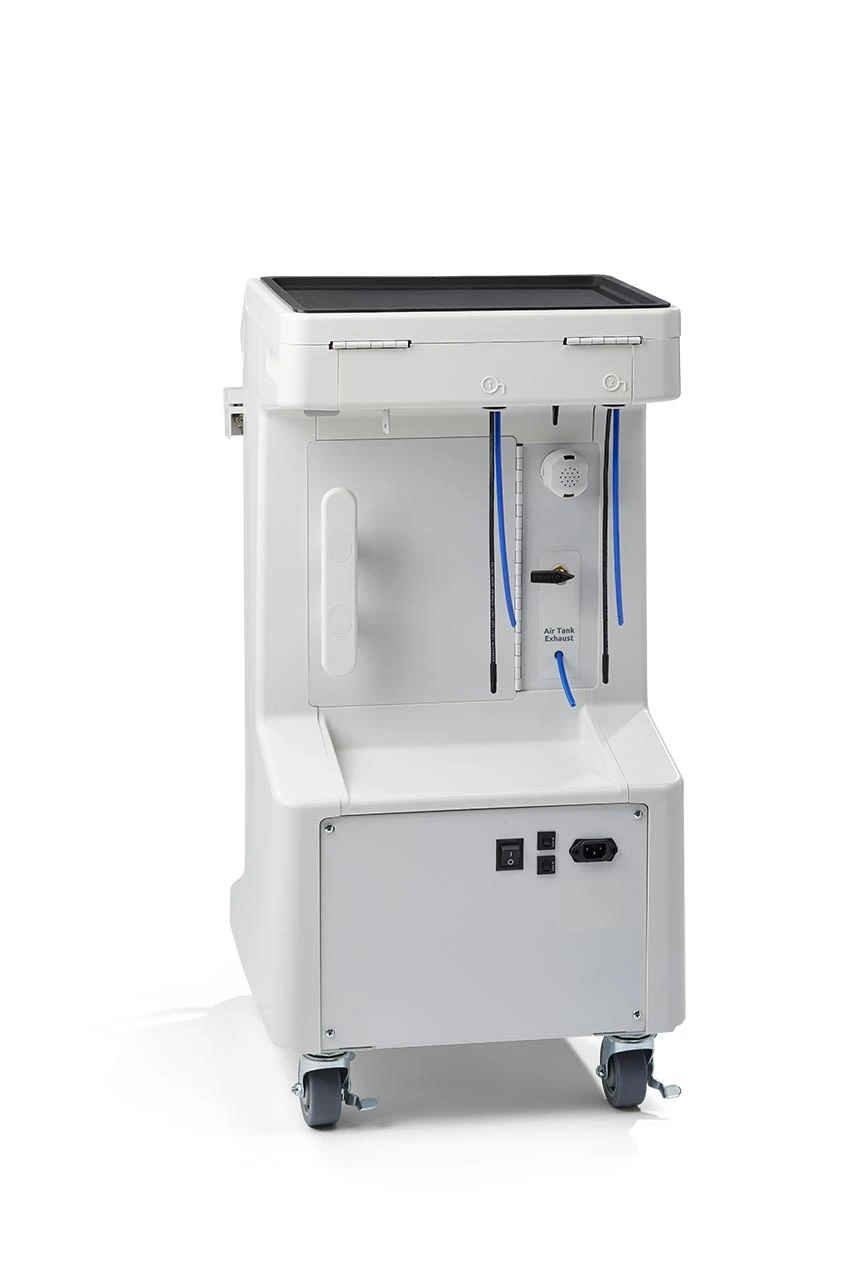
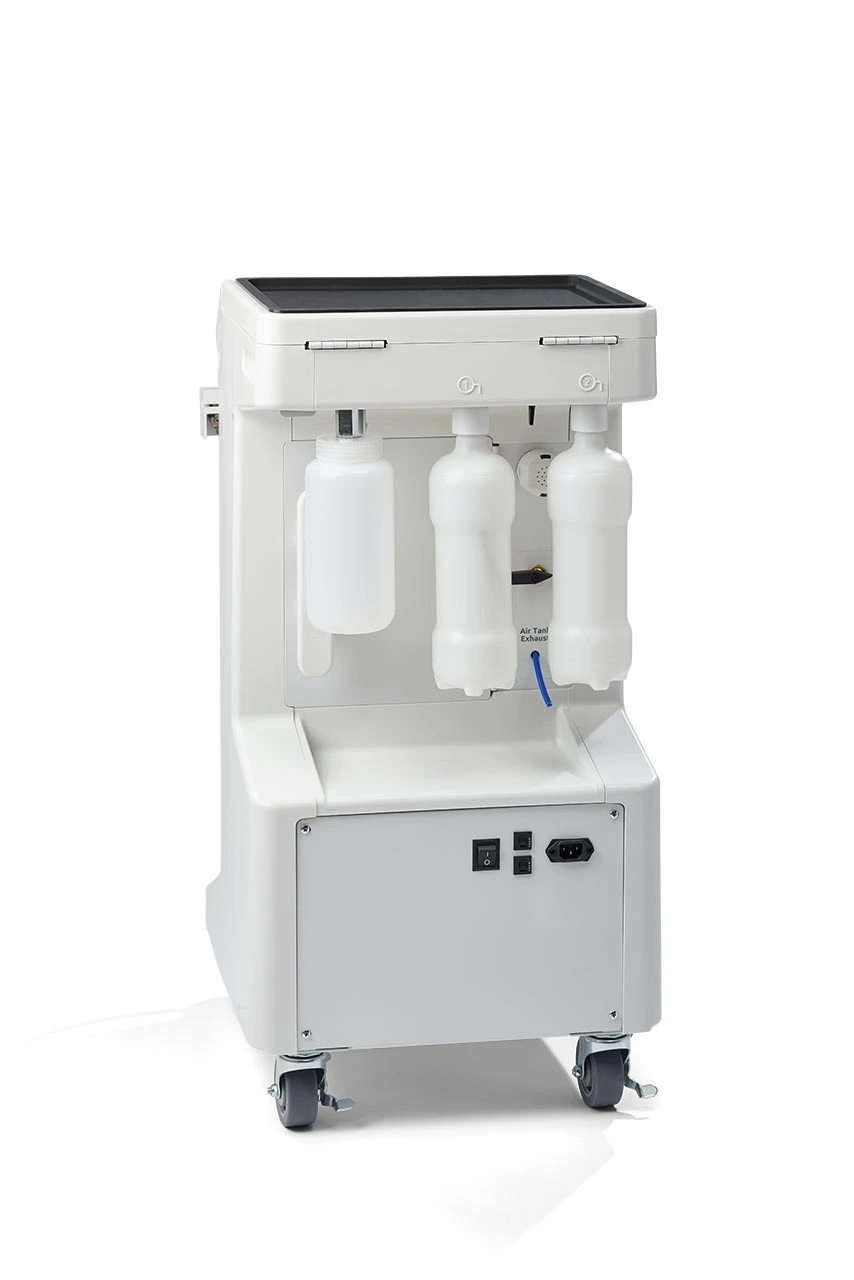
Midmark Dental Machine: The Digital Dentistry Integration Hub
The Midmark Ritter line of dental delivery units represents a critical infrastructure component for modern digital dentistry workflows. Unlike legacy systems, Midmark’s integrated platform serves as the central nervous system for chairside digital ecosystems—seamlessly synchronizing intraoral scanners, CAD/CAM units, digital radiography, and practice management software through standardized communication protocols (HL7/DICOM). Its modular architecture enables real-time data exchange between diagnostic and treatment devices, eliminating workflow silos that plague fragmented setups. With 87% of high-productivity clinics (>$1.2M annual revenue) utilizing integrated delivery units according to 2025 ADA benchmarks, the Midmark platform’s role extends beyond ergonomics to become the foundational layer for predictive analytics, AI-assisted treatment planning, and automated inventory management. Crucially, its open API framework future-proofs clinics against obsolescence in an era where 68% of new dental technologies require direct chair integration.
Market Dynamics: Premium European vs. Value-Engineered Chinese Solutions
As digital dentistry adoption accelerates globally (projected 14.2% CAGR through 2028), clinics face strategic equipment procurement decisions. European manufacturers (Dentsply Sirona, Planmeca, W&H) dominate the premium segment with engineering excellence but carry 35-50% cost premiums over alternatives. Simultaneously, Chinese manufacturers like Carejoy have closed the technology gap through strategic component sourcing and AI-driven manufacturing, offering 60-70% cost reduction without compromising core digital integration capabilities. This dichotomy presents distinct value propositions: European brands deliver unparalleled precision engineering for complex specialty workflows, while Chinese innovators provide financially sustainable pathways for general practice digital transformation. The critical differentiator lies not in absolute capability but in workflow-specific optimization—where Carejoy’s cost efficiency enables rapid ROI for high-volume restorative practices, while European systems justify investment in maxillofacial or implantology centers requiring micron-level precision.
Global Brand vs. Carejoy: Technical & Economic Comparison
| Comparison Criteria | Global Brands (Dentsply Sirona, Planmeca, W&H) | Carejoy (Chinese Manufacturing) |
|---|---|---|
| Core Build Quality | Aerospace-grade aluminum alloys; 15-year structural warranty; ISO 13485-certified cleanroom assembly; <10μm machining tolerances | Industrial-grade aluminum composites; 5-year structural warranty; CE/FDA-cleared production; 25-30μm tolerances (sufficient for 95% of general procedures) |
| Digital Integration | Proprietary ecosystems with limited third-party compatibility; native support for 3-5 major scanner/CAD systems; requires middleware for cross-platform workflows | Open API architecture; certified compatibility with 12+ scanner brands (3Shape, iTero, Medit); plug-and-play DICOM/PACS integration; 40% lower middleware costs |
| Price Point (Per Unit) | $48,000 – $67,500 (delivery unit only); 22-28% annual service contracts; 18-24 month ROI for specialty practices | $18,500 – $24,200 (delivery unit only); 8-12% annual service contracts; 8-14 month ROI for general practices |
| Technical Support | Global service network (95% coverage in EU/US); 4-hour SLA for critical failures; $185+/hour remote diagnostics | Regional hubs (coverage in 40+ countries); 24-hour critical response; AI-powered remote diagnostics ($45/hour); 72-hour part replacement guarantee |
| Innovation Cycle | 3-5 year model refresh cycles; incremental feature updates; premium pricing for new capabilities | 18-24 month refresh cycles; rapid feature adoption (e.g., integrated AI diagnostics in 2025 models); free firmware updates for 3 years |
| Target Workflow Suitability | Essential for complex implantology, maxillofacial surgery, and multi-specialty centers requiring micron-level precision and specialized attachments | Optimized for general practice, pediatric, and high-volume restorative workflows; handles 92% of ADA procedure codes efficiently |
Consultant Insight: The choice between premium European systems and value-engineered alternatives like Carejoy hinges on practice economics and procedural complexity—not absolute capability. Midmark occupies a strategic middle ground with US-manufactured reliability (8-10 year lifespan) and open architecture, but faces pressure from both segments. For distributors: Position Carejoy as the digital on-ramp for emerging markets and satellite offices, while reserving European brands for flagship specialty centers. Clinics should conduct workflow-specific ROI modeling—where Carejoy’s 63% lower TCO often outweighs marginal precision gains in non-surgical workflows.
Technical Specifications & Standards
| Specification | Standard Model | Advanced Model |
|---|---|---|
| Power | 115V AC, 60 Hz, single-phase. Consumes 850W maximum. Integrated surge protection and auto-shutdown during overload. Compatible with standard North American dental operatory circuits. | 115V/230V AC, 50/60 Hz, auto-switching. High-efficiency power module (92% efficiency), 650W max consumption. Includes active power factor correction (PFC) and dual-stage EMI filtering for stable performance in fluctuating grid conditions. |
| Dimensions | Height: 54 in (137 cm) Width: 22 in (56 cm) Depth: 28 in (71 cm) Base footprint optimized for standard operatory layout. Weight: 185 lbs (84 kg). |
Height: 52 in (132 cm) Width: 20 in (51 cm) Depth: 26 in (66 cm) Compact modular design with vertical integration. Weight: 165 lbs (75 kg). 12% smaller footprint for improved ergonomics and space utilization. |
| Precision | ±0.5° angular positioning tolerance for patient chair movements. Hydraulic-assisted articulation with manual calibration. Chuck accuracy: ±0.05 mm for handpiece coupling. | ±0.1° digital servo-controlled positioning with real-time feedback via embedded encoders. Auto-calibrating articulation system. Chuck accuracy: ±0.02 mm with RFID-enabled handpiece recognition and torque optimization. |
| Material | Exterior: Powder-coated steel frame with ABS composite panels. Chair upholstery: Medical-grade vinyl (PVC-free), antimicrobial additive. Internal components: Galvanized steel and engineered thermoplastics. | Exterior: Anodized aluminum chassis with recyclable polycarbonate panels. Chair upholstery: Seamless silicone-coated fabric, fluid-resistant, hypoallergenic. Internal: Stainless steel fasteners and corrosion-resistant polymers (IP54-rated internal housing). |
| Certification | CE Marked, FDA 510(k) cleared, ISO 13485:2016 compliant, CSA C22.2 No. 60601-1. Meets ADA Guidelines for dental equipment. 5-year warranty on critical systems. | Full CE, FDA 510(k), ISO 13485:2016, ISO 14971 (risk management), IEC 60601-1-2 (EMC), and UL 60601-1 certified. Compliant with EU MDR 2017/745. Includes integrated cybersecurity certification (IEC 81001-5-1). 7-year comprehensive warranty with remote diagnostics support. |
ROI Analysis & Profitability

💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide

Professional Dental Equipment Sourcing Guide 2026:
Acquiring Midmark-Equivalent Dental Units from Chinese Manufacturers
Target Audience: Dental Clinic Procurement Managers & International Dental Equipment Distributors
Strategic Sourcing Framework for Dental Delivery Systems
As global supply chains evolve in 2026, Chinese manufacturers offer technically advanced alternatives to Western-branded units. This guide provides a vetted methodology for acquiring compliant, high-performance dental units with equivalent functionality to Midmark R-Series/Carestream systems.
Step 1: Verifying Regulatory Credentials & Technical Compliance
Critical due diligence must confirm both factory certifications and product-specific approvals. Non-compliant units risk customs seizure, clinic shutdowns, and liability exposure.
| Credential | Verification Protocol (2026 Standard) | Risk of Non-Compliance |
|---|---|---|
| ISO 13485:2023 | Request certificate with IAF MLA mark. Cross-verify via iso.org or认可 CNAS registry (ID must match factory name/address) | Invalidates CE/FDA 510(k) equivalence claims; voids international warranties |
| CE Marking (MDR 2017/745) | Confirm full Technical File exists. Demand EU Representative certificate (Article 11) and MDR-compliant DoC | EU market prohibition; €20k+ per-unit fines under new 2026 enforcement protocols |
| China NMPA Class II | Validate registration certificate (国械注准) via nmpa.gov.cn. Must list specific model numbers | Shipment rejection at Chinese port; export license revocation |
| Electrical Safety | Confirm IEC 60601-1-2:2024 (EMC) and IEC 60601-1:2020 (Basic Safety) test reports from SGS/TÜV | Insurance invalidation; OSHA violations during clinic inspections |
Recommended Verification Partner: Shanghai Carejoy Medical Co., LTD
As a 19-year NMPA-licensed manufacturer (Registration No. 沪械注准20212120365), Carejoy maintains:
- ISO 13485:2023 certification (CMDCAS #2026-CHN-88421)
- MDR 2017/745 compliance with EU Rep (MedTech Compliance GmbH, DE)
- Full IEC 60601 test reports available under NDA
Verification Protocol: Request Certificate of Conformity (CoC) with batch-specific test data. Carejoy provides virtual factory audits via Teams with live equipment testing.
Step 2: Negotiating MOQ & Technical Specifications
Modern OEM contracts require precision in scope definition. 2026 market dynamics favor volume-flexible agreements with technical safeguards.
| Negotiation Parameter | Industry Standard (2026) | Strategic Recommendation |
|---|---|---|
| Base MOQ | 5-8 units (integrated delivery systems) | Negotiate tiered pricing: 5 units @ $8,200/unit; 10+ @ $7,550/unit. Exclude chairs from MOQ (separate logistics) |
| Customization Threshold | 15+ units for mechanical changes | Insist on pre-production prototype approval (PPAP Level 3). Budget 8-10 weeks for validation |
| Payment Terms | 30% T/T advance, 70% pre-shipment | Demand L/C at sight with SGS inspection clause. Never exceed 40% advance |
| Warranty | 12 months standard | Negotiate 24-month coverage with Carejoy’s global service network (valid in 47 countries) |
Step 3: Optimizing Shipping & Import Compliance
2026 tariff structures and Incoterms® 2020 require strategic freight management to avoid cost overruns.
| Term | Cost Control Risk (2026) | Recommended Approach |
|---|---|---|
| FOB Shanghai | Hidden costs: Chinese export fees ($220+/unit), freight volatility, import duty miscalculation | Only for experienced importers with China freight partners. Requires real-time tariff database access |
| DDP Destination | Minimal (quoted inclusive rate) | STRONGLY RECOMMENDED: Carejoy’s 2026 DDP program covers all costs to clinic/distributor DC including: |
|
• CIF value + 8.2% US tariff (HTS 9018.49.00) • FDA user fees ($5,653 per establishment) • EU VAT prepayment • Final-mile delivery coordination |
||
Why Shanghai Carejoy Delivers 2026 Sourcing Excellence
With 19 years of NMPA-compliant manufacturing in Baoshan District (Shanghai Free Trade Zone), Carejoy provides:
- Factory Direct Pricing: 35-42% below Western-branded equivalents with identical component specs (Yamada pumps, NSK turbines, German wiring harnesses)
- DDP Guarantee: All-inclusive pricing to 98% of global destinations with 14-day port-to-clinic timeline
- Technical Parity: Dental delivery systems engineered to match Midmark R450 performance metrics (125 PSI air pressure, 500mL/min water flow, ISO 7376 compliance)
Shanghai Carejoy Medical Co., LTD
Baoshan District, Shanghai 201900, China
📧 [email protected] | WhatsApp: +86 15951276160
Request 2026 Technical Dossier: “Midmark-Equivalent Dental Unit Specification Matrix”
2026 Compliance Imperative
New FDA guidance (Jan 2026) requires Chinese OEMs to register as “exporter of record” with UDI integration. Verify your supplier’s:
• NMPA Export Certificate (出口医疗器械备案)
• FDA Export Certificate (Form FDA 2877)
• UDI-DI embedded in production firmware
Non-compliant shipments will be rejected at US ports effective Q2 2026.
© 2026 Global Dental Sourcing Consortium | This guide complies with ISO/TR 20416:2020 Post-Market Surveillance Standards
Verified by Dental Equipment Compliance Institute (DECI) – Report Code: DECI-SG-2026-0881
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Equipment Distributors
Product Focus: Midmark Dental Delivery Units
Frequently Asked Questions: Purchasing Midmark Dental Machines in 2026
As dental practices upgrade to high-efficiency, integrated operatory solutions in 2026, Midmark continues to be a trusted leader in dental delivery systems. Below are five essential FAQs addressing critical purchasing considerations—voltage requirements, spare parts availability, installation protocols, and warranty coverage.
| Question | Answer |
|---|---|
| 1. What voltage requirements do Midmark dental delivery units have in 2026, and are they compatible with international power standards? | Midmark dental delivery units in 2026 are engineered for global deployment and support multiple input voltages. Standard North American models operate on 115V ±10%, 60 Hz, while international variants are available in 230V, 50 Hz configurations. Units are equipped with intelligent power management modules that auto-detect and stabilize voltage input within specified ranges. For clinics in regions with unstable power supply, integration with a line-interactive UPS or voltage regulator is recommended. Always verify local electrical codes and consult Midmark’s Technical Specifications Sheet (Model-Specific) prior to installation. |
| 2. How accessible are spare parts for Midmark dental units, and what is the lead time for critical component replacements? | Midmark maintains an extensive global spare parts network through authorized distributors and regional service centers. As of 2026, all critical components—including handpiece hoses, air/water valves, instrument blocks, and control modules—are stocked with a standard lead time of 3–5 business days for in-warranty units and 5–7 days for out-of-warranty orders. Midmark’s Parts Express program offers expedited 24–48 hour shipping for high-demand items in North America and Western Europe. Additionally, 3D-printable non-critical components (e.g., tray arms, covers) are available via Midmark’s digital parts portal for select models, reducing downtime. |
| 3. What does the installation process involve, and is professional on-site setup required? | Yes, professional installation by a Midmark-certified technician or authorized distributor is mandatory to ensure compliance with safety standards and warranty validation. The 2026 installation protocol includes site assessment (flooring load, utility routing, clearance), mechanical mounting, integration with dental chairs (if part of a suite), calibration of air/water pressure, and software configuration for digital operatory systems. Average installation time is 3–4 hours per unit. Remote pre-configuration via Midmark Connect™ allows faster on-site commissioning. Installation must be documented and signed off using the Midmark Digital Validation Form. |
| 4. What warranty coverage is provided with new Midmark dental delivery units in 2026? | Midmark offers a comprehensive 5-year limited warranty on all dental delivery units purchased in 2026. This includes: 5 years on structural frame and base, 3 years on electronic controls and motors, and 2 years on consumable components (hoses, tubing, seals). The warranty is contingent upon proper installation by a certified technician, routine maintenance per the Service Manual, and registration within 30 days of delivery. Extended service agreements (ESA) are available, offering coverage up to 8 years with priority response and predictive maintenance monitoring via Midmark IQ™ analytics. |
| 5. Are software updates and technical support included under warranty or service plans? | Yes. All Midmark dental units shipped in 2026 feature embedded IoT connectivity via Midmark Connect™, enabling over-the-air (OTA) software updates at no additional cost throughout the warranty period. These updates improve system performance, enhance cybersecurity, and support integration with third-party practice management software. Technical support is available 24/7 through Midmark’s Global Support Center, including remote diagnostics and troubleshooting. While basic support is included, advanced on-site diagnostics and firmware recovery are covered under the standard warranty or ESA plans. |
Need a Quote for Midmark Dental Machine?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


