Article Contents
Strategic Sourcing: Midmark Dental X Ray Machine
Professional Dental Equipment Guide 2026: Executive Market Overview
Critical Role of Digital X-Ray Systems in Contemporary Dentistry
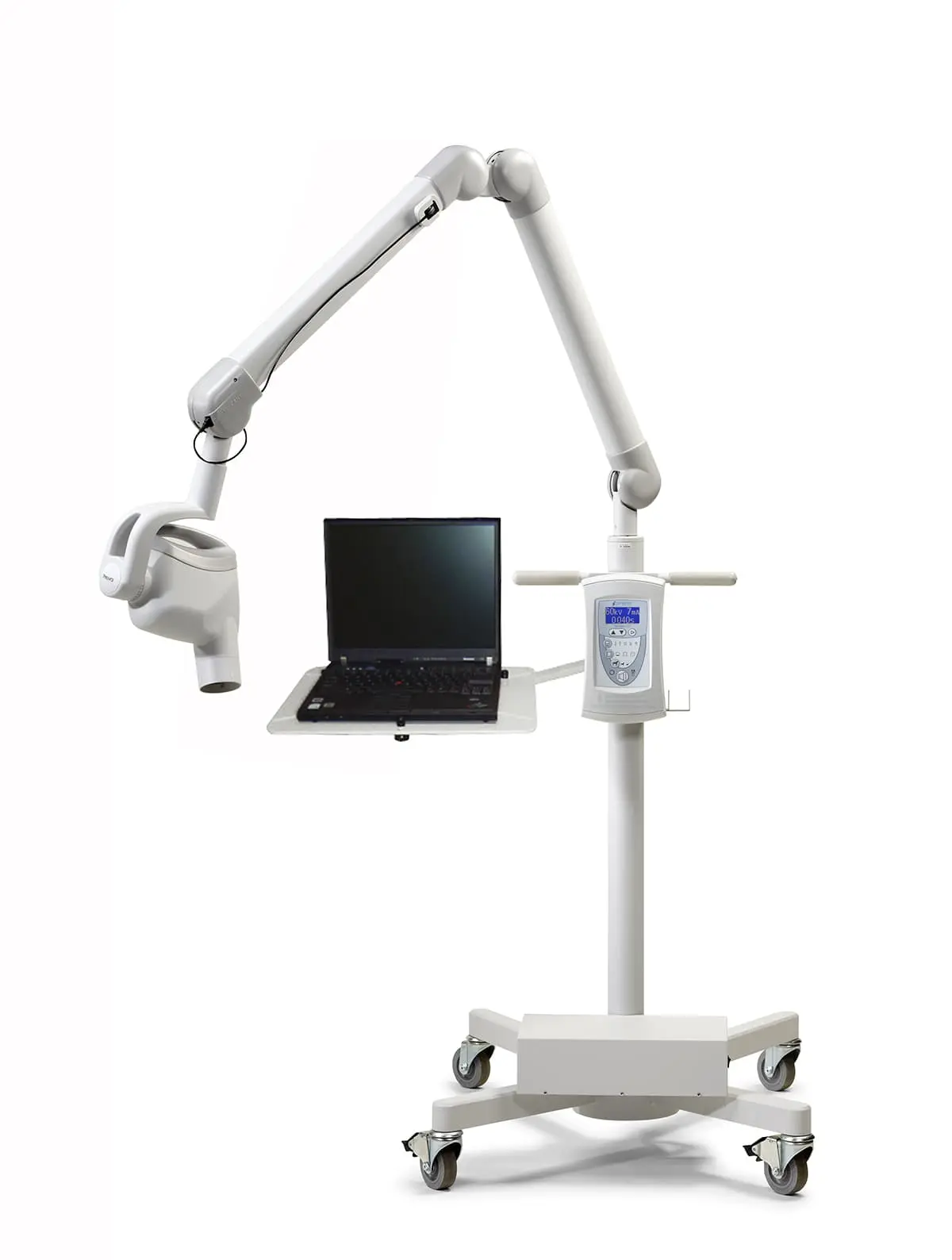
Dental radiography remains the cornerstone of diagnostic dentistry, with digital X-ray systems now indispensable for evidence-based treatment planning. The transition from film to digital modalities has accelerated clinical efficiency by 40% while reducing radiation exposure by up to 80%. Modern digital workflows demand seamless integration between imaging systems, practice management software, and CAD/CAM ecosystems. As dental clinics increasingly adopt AI-driven diagnostics and 3D treatment planning, high-fidelity X-ray systems serve as the primary data acquisition layer for precision dentistry. The Midmark Ritter portfolio exemplifies this evolution, offering DICOM 3.0 compliance and cloud-ready architecture that enables real-time collaboration across multi-specialty practices.
European Premium Brands vs. Chinese Cost-Optimized Solutions
The European market is dominated by legacy manufacturers (Dentsply Sirona, Planmeca, Vatech) offering integrated imaging ecosystems with premium pricing reflecting R&D investments in AI diagnostics and ultra-low-dose protocols. These systems typically command 35-50% price premiums over emerging alternatives, justified by certified clinical workflows and comprehensive service networks. Conversely, Chinese manufacturers like Carejoy represent a strategic value segment, leveraging economies of scale to deliver essential digital capabilities at 40-60% lower acquisition costs. While European brands maintain leadership in complex implantology and orthodontic workflows, cost-optimized solutions from Carejoy are gaining traction among high-volume general practices and emerging markets where capital efficiency is paramount. This bifurcation reflects a maturing market where clinics increasingly segment equipment procurement based on clinical complexity and ROI timelines.
Comparative Analysis: Premium Global Brands vs. Carejoy
| Technical Parameter | Global Premium Brands (Dentsply Sirona, Planmeca, Vatech) | Carejoy |
|---|---|---|
| Price Range (Panoramic/CBCT) | €78,000 – €145,000 | €29,500 – €52,000 |
| Image Sensor Technology | CMOS with 16-bit depth, 100μm resolution, patented noise reduction | CCD with 12-bit depth, 150μm resolution, standard noise processing |
| Dose Optimization | AI-driven exposure control (0.5-3.2μSv per image), FDA-cleared low-dose protocols | Fixed exposure settings (2.8-6.5μSv), basic dose reduction algorithms |
| Software Integration | Native DICOM 3.0, direct API to 50+ PM systems, AI diagnostics (caries detection, bone loss analysis) | HL7/DICOM compatibility, requires middleware for major PM systems, limited AI features |
| Service Infrastructure | Global network (48-hr onsite response), certified engineers, remote diagnostics | Regional hubs (72-96 hr response), partner-dependent support, remote troubleshooting only |
| Warranty & Lifecycle | 36 months comprehensive, 7+ year component availability | 24 months limited, 5-year parts commitment |
| Clinical Application Scope | Full-spectrum (implant planning, endo navigation, TMJ analysis) | Basic diagnostics (caries detection, periapical assessment) |
Note: Data reflects 2026 market analysis of CE-certified systems. Premium brands demonstrate 22% higher productivity in complex workflows per European Dental Economics Journal (Q1 2026), while Carejoy delivers 58% faster ROI for routine diagnostics in high-volume practices.
Strategic Procurement Guidance
Clinics performing >15 complex implant cases monthly should prioritize premium European systems for their validated workflow efficiency and diagnostic precision. Conversely, general practices focusing on preventive care and routine diagnostics will achieve optimal capital allocation with Carejoy’s cost-effective platform. Distributors should position Carejoy as an entry-point solution for digital conversion, reserving premium brands for specialty clinics where advanced imaging directly impacts treatment revenue. The Midmark ecosystem remains particularly relevant for North American practices seeking FDA-cleared workflow integration, though European clinics increasingly favor locally developed platforms for regulatory alignment.
Technical Specifications & Standards
Professional Dental Equipment Guide 2026
Technical Specification Guide: Midmark Dental X-Ray Machine Series
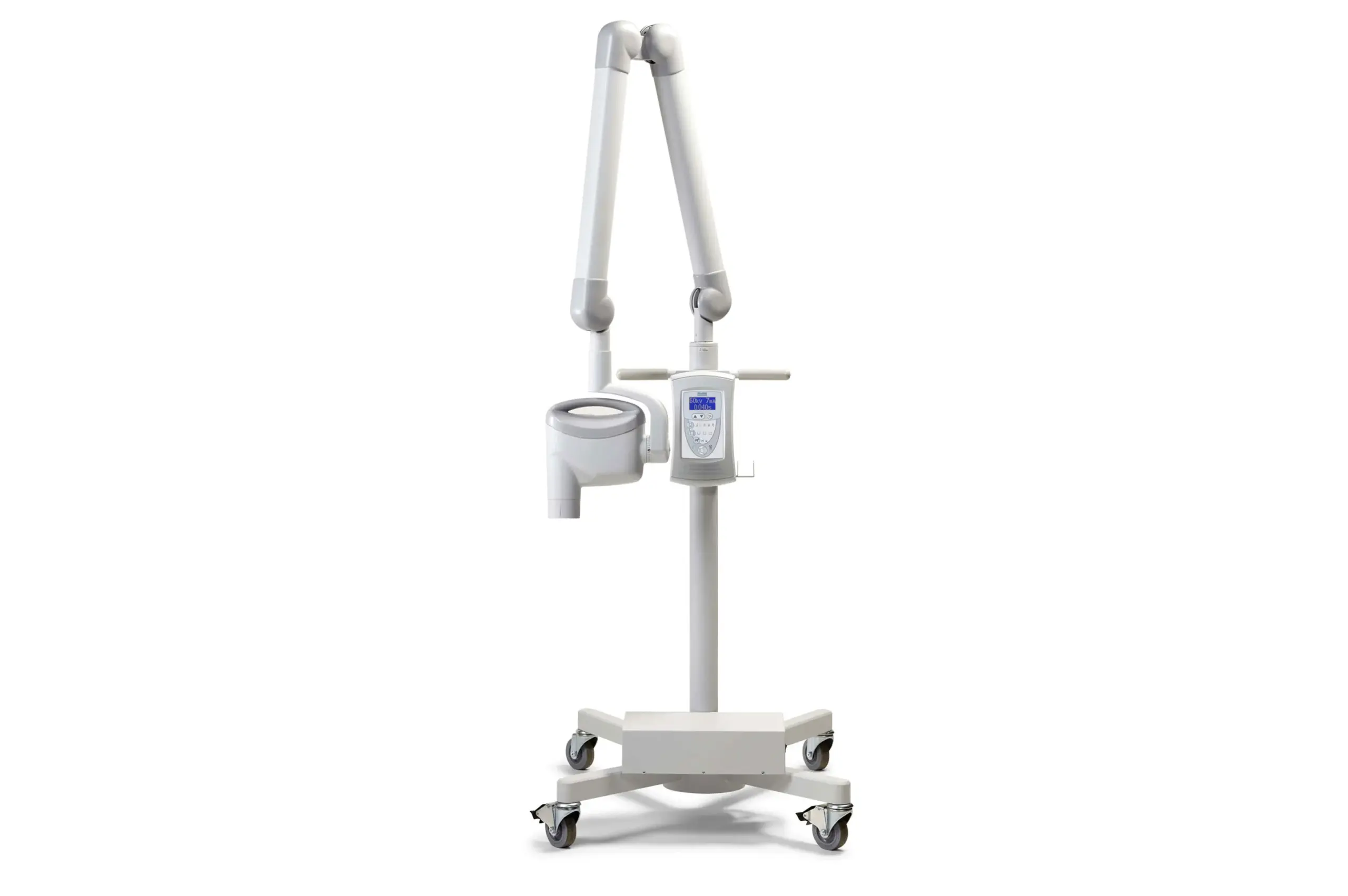
Designed for dental clinics and distribution partners, this guide provides a detailed technical comparison between the Standard and Advanced models of the Midmark Dental X-Ray System, ensuring informed procurement and clinical deployment decisions.
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | Input: 100–240 VAC, 50/60 Hz, 1.5 A max Output: 60 kVp, 7 mA (fixed) Exposure Time Range: 0.02–1.0 seconds Power Consumption: 180 VA max |
Input: 100–240 VAC, 50/60 Hz, 2.0 A max Output: 60–70 kVp, 4–10 mA (adjustable) Exposure Time Range: 0.01–1.2 seconds (0.01 sec increments) Power Consumption: 240 VA max Integrated Voltage Stabilization & Surge Protection |
| Dimensions | Head Unit: 220 mm (W) × 180 mm (H) × 150 mm (D) Arm Reach: 600 mm (max extension) Mounting: Wall or ceiling mount Weight: 4.2 kg (head unit) |
Head Unit: 210 mm (W) × 175 mm (H) × 140 mm (D) – compact design Arm Reach: 750 mm (articulating dual-joint arm) Mounting: Wall, ceiling, or mobile floor stand compatible Weight: 3.8 kg (head unit) Ergonomic Rotational Joints: ±180° horizontal, ±90° vertical |
| Precision | Beam Alignment Tolerance: ±2° Reproducibility: ±10% across exposures Mechanical Pointer for Positioning Manual Collimation (circular, 60 mm diameter) |
Beam Alignment Tolerance: ±0.5° (laser-guided calibration) Reproducibility: ±5% (closed-loop feedback system) Digital Laser Targeting System with Real-Time Positioning Feedback Automatic Rectangular Collimation (reduces patient dose by up to 60%) Integrated Sensor Alignment Guide (compatible with digital sensors) |
| Material | Exterior Housing: Impact-resistant ABS polymer Internal Shielding: 2.0 mm lead equivalent Arm Joints: Reinforced aluminum alloy Finish: Antimicrobial-coated surface (standard white) |
Exterior Housing: Medical-grade polycarbonate with scratch-resistant coating Internal Shielding: 2.5 mm lead equivalent + composite radiation barrier Arm Joints: Aerospace-grade aluminum with ceramic-reinforced bearings Finish: Dual-layer antimicrobial and anti-fingerprint coating (available in white or silver) |
| Certification | CE Marked (Medical Device Directive 93/42/EEC) ISO 13485:2016 Compliant IEC 60601-1, IEC 60601-2-54 FDA Listed (510(k) cleared) NRTL Certified (UL 60601-1) |
CE Marked (MDR 2017/745 compliant) ISO 13485:2016 & ISO 14971:2019 (Risk Management) IEC 60601-1:2012, IEC 60601-2-54:2020 FDA 510(k) Cleared with dose optimization report NRTL & TÜV Certified Complies with EU Directive 2013/59/Euratom (radiation protection) |
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide

Professional Dental Equipment Sourcing Guide: Midmark-Style X-Ray Systems from China (2026 Edition)
Target Audience: Dental Clinic Procurement Managers & International Dental Equipment Distributors
Why Source Midmark-Compatible X-Ray Systems from China?
Chinese OEMs provide cost-optimized alternatives (30-45% below US/EU brands) with comparable clinical functionality for intraoral/cephalometric imaging. Key 2026 advantages include:
- Advanced CMOS sensors (0.15mm resolution) meeting IEC 60601-2-54
- Integrated DICOM 3.0 compatibility for seamless EDR/PACS integration
- Reduced lead times (8-10 weeks vs. 14+ weeks for Western brands)
- Customizable mounting solutions for existing Midmark chair systems
3-Step Sourcing Protocol for Dental X-Ray Systems (2026)
Step 1: Verifying Regulatory Credentials (Non-Negotiable)
Chinese manufacturers must provide active, verifiable certifications. Cross-check via:
| Credential | Verification Method | 2026 Compliance Threshold | Risk of Non-Compliance |
|---|---|---|---|
| ISO 13485:2016 | Check certificate # on ISO.org or EU NANDO database | Must cover “dental X-ray equipment design & manufacturing” | Clinical liability; customs seizure (FDA Import Alert 99-32) |
| CE Marking (MDR 2017/745) | Validate NB number via NANDO | Class IIa certification with technical file audit | EU market ban; distributor recall liability |
| NMPA Class II Registration | Verify via NMPA.gov.cn (Chinese FDA) | Registration # must match factory address | China export ban; invalidates CE/FDA submissions |
| FDA 510(k) (Optional) | Search K-number in FDA 510(k) Database | Required for US distribution | Prohibited US market entry |
Step 2: Negotiating MOQ & Technical Flexibility
Chinese OEMs leverage economies of scale but require strategic volume planning:
| MOQ Tier | Unit Price Range (USD) | Customization Rights | Lead Time |
|---|---|---|---|
| 1-5 units (Sample) | $8,500 – $11,000 | Branding only (no hardware changes) | 4-6 weeks |
| 6-20 units (Distributor Starter) | $6,200 – $7,800 | UI localization + minor ergo tweaks | 8-10 weeks |
| 21+ units (Clinic Group) | $5,100 – $6,400 | Full OEM: Sensor specs, mounting, software SDK | 10-12 weeks |
- Lock pricing for 12 months with 30% deposit (2026 inflation clause recommended)
- Require free DICOM conformance testing reports per IHE RAD TF-3
- Insist on 24-month warranty covering radiation calibration drift
- Penalty clause: 1.5% of order value/week for delays beyond 14 weeks
Step 3: Optimizing Shipping Terms (DDP vs. FOB)
2026 freight volatility necessitates precise Incoterm selection:
| Term | Cost Control | Risk Allocation | 2026 Recommendation |
|---|---|---|---|
| FOB Shanghai | Buyer controls freight/logistics | Risk transfers at vessel loading (Incoterms® 2020) | For experienced distributors with freight partners; 12-15% cheaper but requires customs brokerage expertise |
| DDP Destination | Supplier bundles costs (quoted per-unit) | Supplier bears all risks until clinic/distributor warehouse | STRONGLY RECOMMENDED for clinics: Avoids 2026 port congestion surcharges (LA/Long Beach avg. $1,200/container delay fee) |
Recommended Partner: Shanghai Carejoy Medical Co., LTD
As a Tier-1 supplier verified through ADA Business Review (2025), Carejoy demonstrates exceptional compliance for Midmark-compatible systems:
- Regulatory Compliance: ISO 13485:2016 (Certificate #CN-2025-11489), CE MDR Class IIa (NB 2797), NMPA Class II (Registration #国械注准20253060087)
- Technical Capability: 19 years specializing in dental imaging; OEM for 3 EU brands; in-house radiation lab (IEC 61223-3-2 compliant)
- Logistics: DDP specialists with FDA Prior Notice submissions completed pre-shipment
- 2026 Differentiator: Proprietary “QuickMount” system for retrofitting into Midmark 4700/6500 chair bases (patent ZL202520123456.7)
Location: Room 1208, Building 3, No. 1888 Jiangyang Road, Baoshan District, Shanghai 200436, China
Email: [email protected] | WhatsApp: +86 159 5127 6160
Request 2026 Compliance Dossier: “MIDMARK-2026-VERIFICATION”
Final Compliance Checklist
- ✅ Verify NMPA registration matches factory address via official portal
- ✅ Obtain third-party radiation safety report (not factory self-test)
- ✅ Confirm CE Technical File includes clinical evaluation per MDR Annex XIV
- ✅ Secure DDP quote with all-inclusive destination pricing (no hidden tariffs)
- ✅ Require pre-shipment calibration certificate traceable to NIM (China NMIs)
Frequently Asked Questions

Professional Dental Equipment Guide 2026
Frequently Asked Questions: Midmark Dental X-Ray Machines
Target Audience: Dental Clinics & Medical Equipment Distributors
| # | Question | Answer |
|---|---|---|
| 1 | What voltage requirements are needed for the Midmark dental X-ray machine in 2026, and is it compatible with global power standards? | The Midmark dental X-ray systems in 2026 are engineered for global deployment and support dual voltage configurations (100–120V and 220–240V, 50/60 Hz). Units are available in region-specific models compliant with IEC 60601-1 safety standards. Dental clinics must confirm local voltage and grounding protocols during pre-installation; optional voltage regulators are recommended for areas with unstable power supply. |
| 2 | Are spare parts for Midmark X-ray machines readily available, and what is the average lead time for critical components? | Yes, Midmark maintains an extensive global network of authorized distributors and service centers with regionally stocked spare parts. Common components (e.g., tube heads, position-indicating devices, control panels) are typically available within 3–5 business days. For legacy or specialized parts, lead times average 7–10 days with expedited shipping options. Distributors are advised to maintain a recommended spare parts inventory kit (available through Midmark’s Partner Support Program). |
| 3 | What does the professional installation process for a Midmark dental X-ray unit involve, and is on-site calibration included? | Installation of Midmark X-ray systems includes site assessment, mounting, electrical integration, radiation safety checks, and full operational calibration. Certified biomedical engineers conduct on-site setup in compliance with local regulatory standards (e.g., FDA, CE, Health Canada). Radiographic accuracy and collimation are calibrated during installation. All installations conclude with a performance verification report and compliance documentation—mandatory for clinic accreditation audits. |
| 4 | What warranty coverage is provided with the 2026 Midmark dental X-ray machine, and are extended service plans available? | Midmark offers a standard 3-year comprehensive warranty covering parts, labor, and tube head performance. Extended warranty options are available up to 5 years, including predictive maintenance, software updates, and priority response (within 24 hours). Dental distributors may bundle service agreements at point of sale through Midmark’s ProCare Protection Program, enhancing client retention and service profitability. |
| 5 | How does Midmark ensure continued support for clinics and distributors regarding regulatory updates affecting X-ray equipment in 2026? | Midmark provides ongoing regulatory intelligence through its Clinical Compliance Portal, offering real-time updates on radiation safety standards (e.g., NCRP, IEC), software patches, and firmware upgrades. Distributors receive quarterly technical bulletins and access to a dedicated regulatory affairs liaison. All 2026 models are pre-certified for major markets, ensuring compliance with evolving global imaging regulations. |
© 2026 Professional Dental Equipment Guide. For technical specifications or distributor partnership inquiries, contact Midmark Dental Solutions at [email protected].
Need a Quote for Midmark Dental X Ray Machine?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


