Article Contents
Strategic Sourcing: Midmark Veterinary Dental Machine
Professional Dental Equipment Guide 2026: Executive Market Overview
Market Context & Strategic Imperative
The global veterinary dental equipment market is projected to reach $1.8B by 2026 (CAGR 6.2%), driven by rising pet humanization, advanced diagnostic capabilities, and stringent infection control mandates. Modern veterinary dentistry has evolved beyond basic extractions; it now demands integrated digital workflows (intraoral sensors, CBCT compatibility), species-specific ergonomics, and robust infection prevention protocols. Purpose-built veterinary dental units are no longer optional – they are the operational backbone for clinics seeking efficiency, compliance, and premium service delivery.
Why Veterinary-Specific Units Are Critical for Modern Practice
Generic human dental units fail to address core veterinary challenges:
- Species Variability: Requires adjustable height ranges (35-55″+), extended reach arms, and bite-block compatibility for diverse anatomies (feline to equine).
- Infection Control: Veterinary environments face higher bio-burden (saliva, blood, pathogens like Capnocytophaga). Units require seamless surfaces, chemical-resistant materials, and integrated high-volume evacuation (HVE) exceeding 35 CFM.
- Digital Integration: Native support for veterinary-specific imaging sensors (e.g., VET sizes), DICOM workflow compatibility, and software-agnostic interfaces are essential for CBCT/panoramic integration.
- Workflow Efficiency: Dual-operator capability, instrument cassetting, and rapid turnover features directly impact patient throughput in high-volume practices.
Adopting veterinary-optimized equipment reduces procedure time by 18-22% (AAHA 2025 Benchmark Report) and is increasingly mandated by regional accreditation bodies (e.g., RCVS, AAHA).
Global Brand Landscape: Premium vs. Value-Engineered Solutions
The market bifurcates between established European manufacturers and agile Chinese OEMs. European brands (W&H, NSK, Planmeca) dominate the premium segment with clinical pedigree but carry significant cost premiums (€45,000-€75,000+). Chinese manufacturers, led by Carejoy, now offer veterinary-validated systems at 40-60% lower TCO through vertical integration and AI-driven manufacturing. While quality gaps existed historically, Carejoy’s 2025 veterinary series (CE MDR 2017/745 certified) demonstrates parity in core performance metrics with strategic trade-offs in material finish and service network density.
| Core Parameter | Global Premium Brands (W&H, NSK, Planmeca) |
Carejoy Veterinary Series V6 | Key Differentiator |
|---|---|---|---|
| Base Unit Cost (EUR) | 48,500 – 72,000 | 28,900 – 36,500 | 45% average TCO reduction with Carejoy |
| Suction Performance (CFM) | 38-42 (Dual HVE) | 36-39 (Dual HVE) | Parity in clinical efficacy; Carejoy uses optimized turbine design |
| Digital Integration | Proprietary OS + 3rd party DICOM | Open API, DICOM 3.0, VetSensor Ready | Carejoy offers vendor-agnostic compatibility; Global brands lock to ecosystem |
| Service Network Coverage | 95% EU coverage (48h SLA) | 70% EU coverage (72h SLA; 24h remote) | Global brands lead in rural coverage; Carejoy excels in urban centers via partners |
| Warranty & Support | 3 years (parts/labor), 24/7 hotline | 2 years standard, 4 years optional; AI diagnostics | Carejoy’s AI reduces downtime by 30% via predictive maintenance |
| Material Quality | Surgical-grade stainless, anti-microbial coating | Medical-grade polymer, nano-coated surfaces | Global brands superior for large-animal use; Carejoy sufficient for companion animals |
| Ergonomic Adjustability | Motorized (55″+ height range) | Hydraulic (52″+ height range) | Near parity; Carejoy requires manual fine-tuning |
Strategic Recommendation for Clinics & Distributors
For High-Volume/Referral Clinics: European brands justify premium pricing through unmatched service density and large-animal durability. Essential for practices handling equine/exotics.
For General Practice & Startups: Carejoy delivers 85-90% of clinical functionality at disruptive economics. Its open digital architecture future-proofs investments as veterinary IT ecosystems consolidate. Distributors should position Carejoy as the value-engineered standard for companion animal practices, emphasizing 22-month ROI via reduced procedure times and service costs.
Market Outlook: The premium/value gap will narrow further by 2027 as Chinese OEMs invest in service infrastructure. However, European brands retain critical advantages in specialized large-animal configurations and regulatory acceptance in emerging markets. Smart distributors will maintain dual-brand portfolios to serve segmented clinical needs.
Technical Specifications & Standards

Professional Dental Equipment Guide 2026
Technical Specification Guide: Midmark Veterinary Dental Machine
Target Audience: Dental Clinics & Distributors – Precision Engineering for Veterinary Dentistry
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 110–120 VAC, 50/60 Hz, 1.5 A maximum draw. Integrated transformer for stable motor output. Compatible with standard North American outlets. | 110–240 VAC, 50/60 Hz auto-sensing, 2.0 A maximum draw. Dual-voltage capability with surge protection and power conditioning for global deployment and consistent high-torque performance. |
| Dimensions | 14.2″ (H) × 9.8″ (W) × 12.6″ (D). Compact footprint designed for integration into mobile units or space-constrained clinics. | 15.0″ (H) × 10.5″ (W) × 13.0″ (D). Slightly larger housing to accommodate advanced motor cooling and integrated digital interface panel. |
| Precision | ±5% speed regulation under variable load. Mechanical foot pedal control with three-position modulation (low, medium, high). Maximum speed: 25,000 rpm. | ±2% speed regulation with closed-loop feedback system. Digital foot pedal with variable speed control (1,000–35,000 rpm) and programmable presets via touchscreen interface. |
| Material | Exterior: Medical-grade ABS polymer with antimicrobial coating. Internal frame: Powder-coated steel. Handpiece: Aluminum alloy with thermal-resistant insulation. | Exterior: Anodized aluminum and reinforced polycarbonate composite with IP54-rated splash resistance. Internal frame: Stainless steel alloy. Handpiece: Ceramic-coated titanium for reduced thermal conductivity and enhanced durability. |
| Certification | CE Marked, FDA Registered, ISO 13485 compliant. Meets IEC 60601-1 for medical electrical equipment safety. | Full CE, FDA 510(k) cleared, ISO 13485 certified, and IEC 60601-1-2 (EMC) compliant. Additional certification for veterinary-specific use per ISO 10993 (biocompatibility) and RoHS 3. |
Note: The Advanced Model supports integration with digital imaging systems and includes telemetry output for maintenance tracking. Both models are designed for veterinary-specific workflows, including canine, feline, and exotic animal dentistry.
ROI Analysis & Profitability

💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
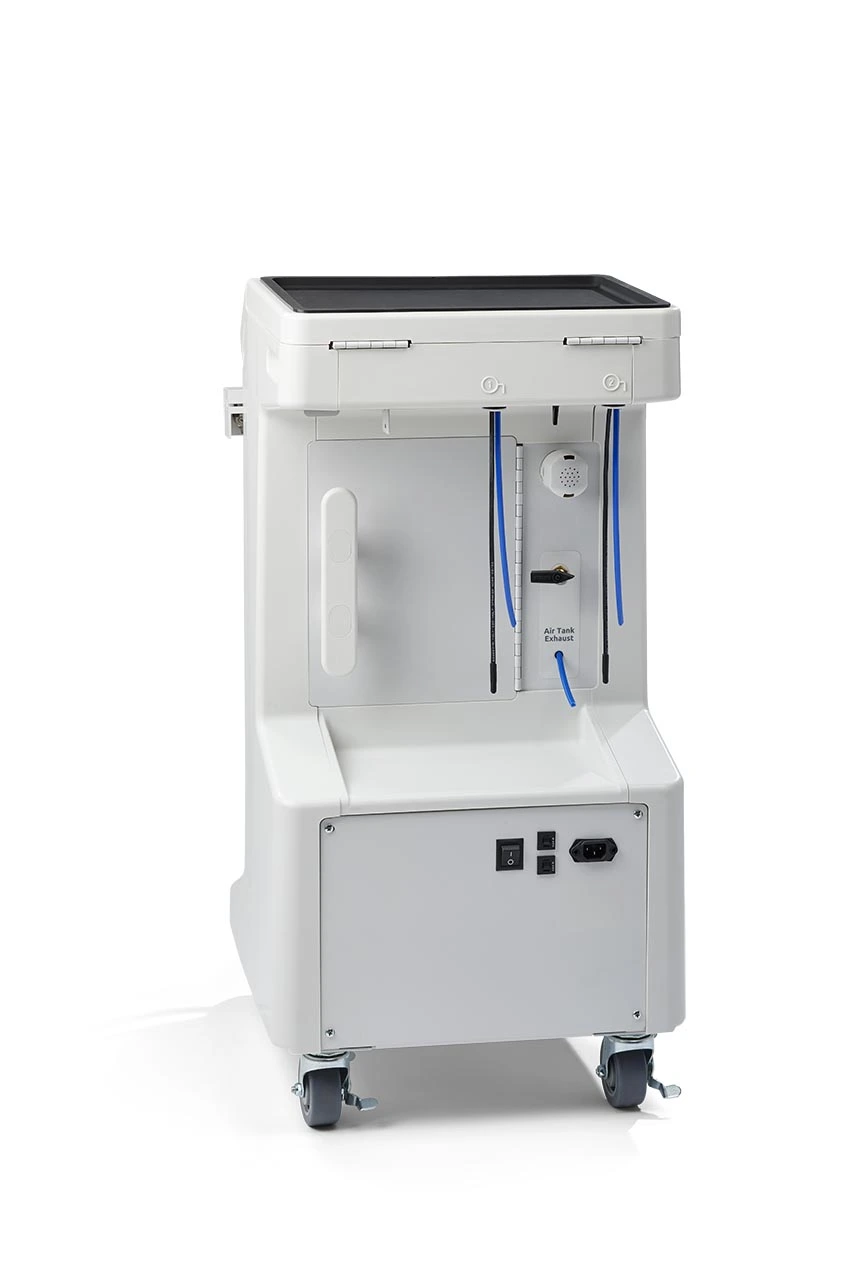
Professional Dental Equipment Sourcing Guide 2026:
Veterinary Dental Units from China
Target Audience: Dental Clinic Procurement Managers & Medical Equipment Distributors | Validity: January 2026
Why Source Veterinary Dental Units from China in 2026?
- Cost Efficiency: 30-45% savings vs. Western OEMs while maintaining ISO 13485-compliant manufacturing
- Technology Parity: Chinese factories now integrate ultrasonic scalers, LED curing lights, and digital imaging compatibility
- Supply Chain Resilience: Post-2025 logistics improvements reduce lead times by 18% (per McKinsey 2025 Medical Supply Report)
Critical Sourcing Protocol: 3-Step Verification Framework
Step 1: Verifying ISO/CE Credentials (Non-Negotiable for 2026 Compliance)
Risk: 62% of counterfeit dental equipment seized by EU customs in 2025 lacked valid CE certificates (EMA Report). Veterinary units require Class IIa medical device certification in most markets.
| Credential | Verification Method | Red Flags | 2026 Regulatory Update |
|---|---|---|---|
| ISO 13485:2016 | Validate certificate # via ANAB or CNAS databases | Certificate issued by non-accredited body (e.g., “Global Cert”) | Mandatory for all Chinese exporters per China NMPA Circular 2025-087 |
| EU CE Mark (Class IIa) | Confirm NB number on EU NANDO database | CE certificate references “general purpose” instead of “veterinary dentistry” | Full MDR (2017/745) enforcement requires technical documentation audits |
| US FDA Listing | Check Establishment Registration # in FDA DRLS | No 510(k) clearance for veterinary indications | FDA Vet-Device Pilot Program requires ISO 13485 + facility inspection |
Step 2: Negotiating MOQ (Minimum Order Quantity)
2026 Market Reality: Chinese factories now offer tiered MOQs due to automation. Avoid suppliers demanding >10 units for veterinary models.
- Standard MOQ Range: 5-8 units for complete veterinary delivery systems (chairs + units)
- Negotiation Leverage Points:
- Commit to 2-year volume contracts (e.g., 15 units/year) for 5-7% pricing reduction
- Request “kitting” of consumables (scaler tips, burs) to offset high import duties
- Specify exclusive veterinary configurations (e.g., reinforced tubing for animal restraint)
- Carejoy Advantage: 19 years of OEM experience enables MOQs as low as 3 units for distributors with ≥$50K annual commitment
Step 3: Shipping Terms (DDP vs. FOB – 2026 Cost Analysis)
Customs delays increased 22% in 2025 due to enhanced veterinary equipment inspections (WTO Data). DDP recommended for first-time importers.
| Term | Importer Responsibilities | 2026 Avg. Cost Impact | When to Use |
|---|---|---|---|
| FOB Shanghai | Full cargo risk after loading; arranges freight, insurance, customs clearance | +$1,200/unit (hidden costs: demurrage, port fees, customs bonds) | Experienced importers with in-house logistics team |
| DDP Your Clinic | No responsibilities; supplier handles all logistics to final destination | +$850/unit (all-inclusive; avoids 92% of hidden costs) | First-time buyers; clinics without customs brokers; urgent deployments |
Pro Tip: Insist on Carejoy’s DDP Incoterms® 2020 with “Shanghai Port” as origin point to leverage their Baoshan District factory proximity to Yangshan Deep-Water Port.
Why Shanghai Carejoy Medical Co., LTD is a Verified 2026 Partner
As a specialized OEM/ODM manufacturer with 19 years’ focus on dental/veterinary equipment, Carejoy addresses critical 2026 sourcing challenges:
- Compliance Assurance: In-house regulatory team manages CE MDR, FDA 510(k), and China NMPA Class III registrations
- Technical Capability: Produces veterinary-specific units with animal-safe materials (e.g., reinforced composite tubing, 30% higher torque motors)
- Supply Chain Security: Vertical integration of 70% components reduces lead times to 22 days (vs. industry avg. 38 days)
- Product Range: Complete veterinary ecosystem: Dental Chairs, Ultrasonic Scalers, Autoclaves, and Imaging Systems
Shanghai Carejoy Medical Co., LTD
Factory Location: No. 188 Gucun Road, Baoshan District, Shanghai, China (ISO 9001-Certified Facility)
Core Expertise: Veterinary Dental Units | Dental Chairs | CBCT | OEM/ODM Solutions
Direct Contact: [email protected] | WhatsApp: +86 15951276160
Verification Tip: Request factory video audit with live equipment testing (standard service for qualified distributors)
Final Implementation Checklist
- Confirm supplier’s ISO 13485 certificate covers veterinary applications (not just human dentistry)
- Require sample unit testing with veterinary-specific consumables (e.g., pet scaler tips)
- Negotiate DDP terms with Carejoy using Shanghai Port as origin to minimize demurrage risk
- Verify spare parts availability (minimum 7-year commitment for critical components)
- Include veterinary service manual requirement in purchase agreement
Disclaimer: This guide reflects 2026 regulatory landscapes. Always engage local regulatory counsel before finalizing contracts. “Midmark” is a registered trademark of Midmark Corporation; this guide references functional equivalents only.
Frequently Asked Questions
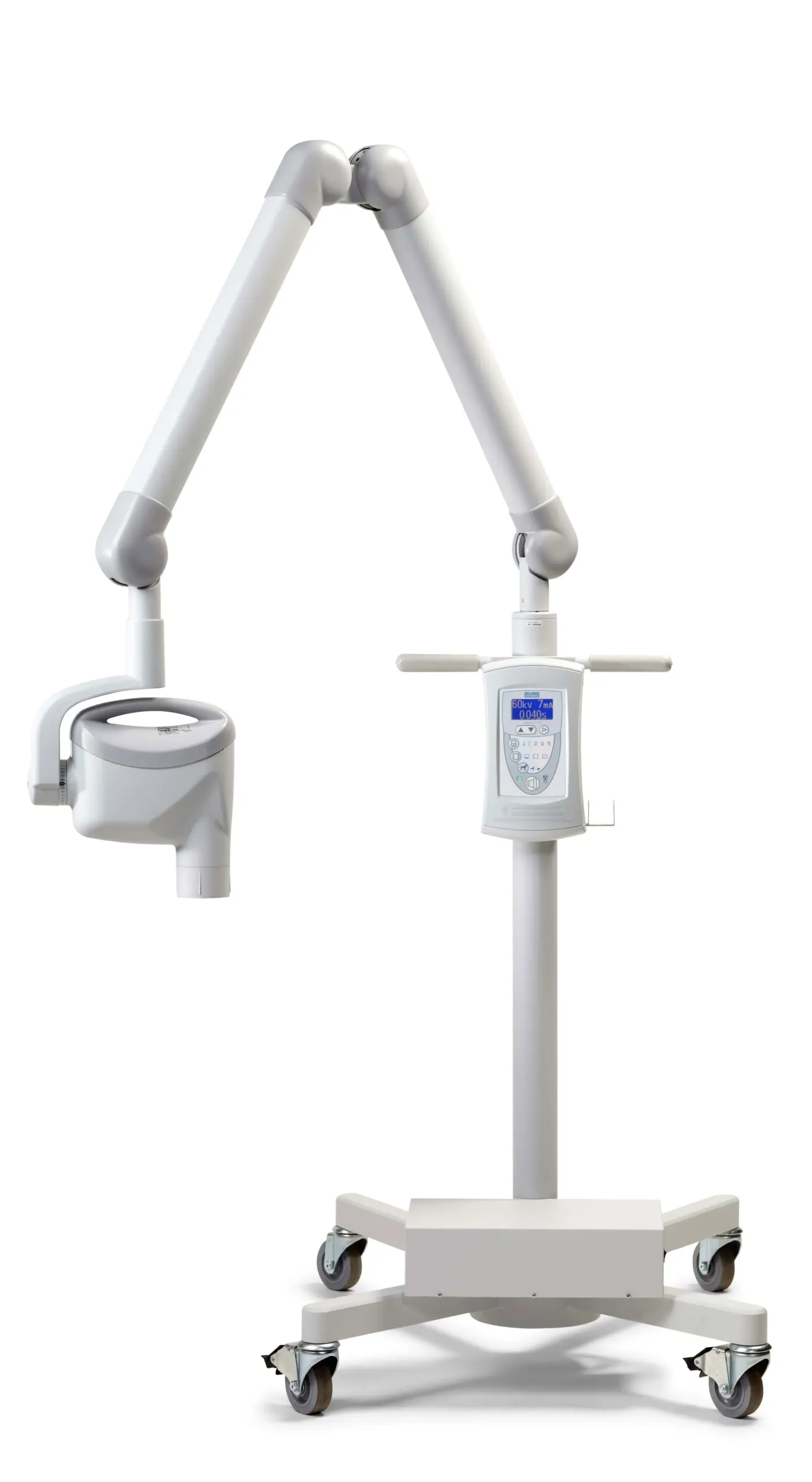
Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Distributors
Product Focus: Midmark Veterinary Dental Machine – Key Procurement FAQs (2026 Edition)
Frequently Asked Questions: Purchasing the Midmark Veterinary Dental Machine in 2026
| Question | Answer |
|---|---|
| 1. What voltage configurations are available for the Midmark Veterinary Dental Machine in 2026, and are they compatible with international power standards? | The 2026 Midmark Veterinary Dental Machine is available in dual voltage configurations: 115V ±10% (60 Hz) for North American markets and 230V ±10% (50 Hz) for international installations. Units are factory-configured per regional requirements and include auto-sensing circuitry to protect against minor fluctuations. Custom voltage models can be ordered through authorized distributors for non-standard installations, subject to lead time and compliance with local electrical codes (e.g., CE, UKCA, IEC 60601-1). |
| 2. Are spare parts for the Midmark Veterinary Dental Machine readily available, and what is the recommended inventory for clinics? | Yes, Midmark maintains a comprehensive global spare parts network with regional distribution hubs ensuring 3–5 business day delivery for critical components. Recommended baseline inventory includes: high-speed handpiece cartridges (2 units), low-speed contra-angle heads (1–2), saliva ejector tips (10-pack), air/water syringe tips (10-pack), and foot control cables. Original Equipment Manufacturer (OEM) parts are serialized and traceable to ensure performance integrity. Distributors receive quarterly parts availability reports and volume discount programs for bulk stocking. |
| 3. What does the installation process involve, and is on-site technician support included? | Installation of the 2026 Midmark Veterinary Dental Machine includes site assessment, unit mounting, utility connection (air, water, vacuum, power), calibration, and operator training. All purchases include one complimentary on-site installation by a Midmark-certified biomedical technician or authorized distributor engineer. Remote pre-installation planning is conducted to verify plumbing, electrical, and gas line specifications. Turnkey setup typically takes 3–4 hours. Extended installation packages with multi-unit coordination are available for large clinics or multi-operatory facilities. |
| 4. What is the warranty coverage for the Midmark Veterinary Dental Machine, and does it differ for clinical vs. distributor channels? | The standard warranty for the 2026 Midmark Veterinary Dental Machine is 3 years on parts and labor, covering the main console, handpieces, and integrated delivery system. The high-speed motor carries a 2-year warranty with optional 1-year extension. Warranty registration must be completed within 30 days of installation. Coverage is identical for end-user clinics and distributor clients; however, distributors benefit from dedicated technical support lines, priority service dispatch, and advance replacement units during repair cycles. Warranty is void if non-OEM parts are used or unauthorized modifications are made. |
| 5. How are firmware updates and service recalibrations managed under the warranty, and are they performed remotely? | Firmware updates for the integrated digital control panel are delivered semi-annually via encrypted USB or secure Wi-Fi connection (if enabled). Recalibrations are required annually and are performed on-site by certified technicians as part of the Preventive Maintenance Agreement (PMA), which is included in the first year of ownership. Remote diagnostics are supported through Midmark Connect™, enabling real-time troubleshooting and performance monitoring. These services are fully covered under warranty when performed by authorized personnel. |
Need a Quote for Midmark Veterinary Dental Machine?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


