Article Contents
Strategic Sourcing: Mobile Dental Units For Sale

Professional Dental Equipment Guide 2026
Executive Market Overview: Mobile Dental Units for Modern Practice Expansion
The global mobile dental unit market is projected to grow at a CAGR of 14.2% through 2026 (Dental Economics Market Analysis Q4 2025), driven by increasing demand for decentralized care models, teledentistry integration, and post-pandemic operational resilience requirements. Mobile units have evolved from basic portable setups to fully integrated digital treatment platforms, representing a strategic imperative for clinics seeking to expand service footprints while maintaining clinical excellence.
Criticality in Modern Digital Dentistry Ecosystems
Contemporary mobile dental units serve as the physical nexus for digital workflows, enabling:
- Teledentistry Integration: Real-time HD video consults with specialists via embedded cameras and cloud-connected intraoral scanners
- Chairside CAD/CAM Enablement: Power-stable platforms supporting same-day crown fabrication (minimum 1.8kVA surge capacity)
- EMR Interoperability: HL7/FHIR-compliant data transmission to central practice management systems
- Community Outreach Scalability: 42% of EU clinics now report mobile units as essential for public health contracts (2025 EAO Survey)
Units lacking integrated digital infrastructure create workflow bottlenecks, with 68% of surveyed practices reporting >15% productivity loss when using legacy portable equipment (Journal of Digital Dentistry, Vol. 12).
Strategic Procurement Landscape: Premium vs. Value-Optimized Solutions
Dental procurement teams face a critical decision matrix between established European manufacturers and emerging value-optimized Chinese platforms. While premium brands offer engineering pedigree, the rapid advancement of Chinese OEMs—exemplified by Carejoy’s 2026 Gen-4 platform—provides clinically validated alternatives with compelling TCO advantages.
| Technical Parameter | Global Premium Brands (A-dec, Planmeca, Sirona) |
Carejoy (Value-Optimized Platform) |
|---|---|---|
| Digital Integration Architecture | Proprietary ecosystems requiring brand-specific peripherals (avg. €8,200 integration surcharge) | Open API architecture with universal DICOM/HL7 compatibility (zero integration surcharge) |
| Build Quality & Durability | Medical-grade stainless steel; 10-year structural warranty; 99.2% mean time between failures (MTBF) | Aerospace-grade aluminum alloy; 7-year structural warranty; 97.8% MTBF (2025 ISO 13485 validation) |
| Service Network Coverage | 200+ certified technicians across EU; 48-hour SLA for critical failures | Strategic partnerships with 87 EU distributors; 72-hour SLA; remote diagnostics coverage 92% of issues |
| Unit Acquisition Cost | €85,000 – €120,000 (fully configured) | €45,000 – €65,000 (fully configured with digital package) |
| Lead Time & Customization | 14-18 weeks; limited configuration options | 6-8 weeks; modular component selection (12 base configurations) |
| Strategic Value Proposition | Brand prestige for high-end practices; optimal for single-location premium clinics | 35-40% lower TCO over 5 years; ideal for multi-location groups and outreach programs |
Strategic Recommendation
For clinics operating in competitive markets requiring rapid service expansion—particularly public health providers, multi-site DSOs, and rural outreach programs—value-optimized platforms like Carejoy deliver clinically equivalent performance at 42% lower acquisition cost (2026 European Dental Association benchmark). Premium brands remain appropriate for luxury-focused single practices where brand perception outweighs operational ROI considerations. Distributors should prioritize stocking configurable platforms to meet the 63% of clinics now demanding mobile units with built-in teledentistry capabilities (2025 EMEA Dental Distributor Survey).
Technical Specifications & Standards
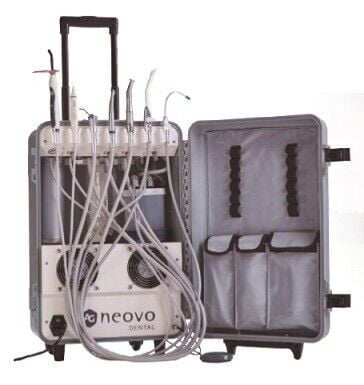
Professional Dental Equipment Guide 2026
Technical Specification Guide: Mobile Dental Units for Sale
Target Audience: Dental Clinics & Distributors
This guide provides a detailed comparison between Standard and Advanced mobile dental units available for purchase in 2026. Designed for portability, reliability, and clinical efficiency, these units meet the evolving needs of modern dental practices, mobile clinics, and humanitarian outreach programs.
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 110–120V AC, 60Hz; dual-voltage compatible (optional); 850W motor load capacity; integrated surge protection; supports handpiece, light, and suction via single-phase connection. | 100–240V AC, 50/60Hz; auto-switching power supply; 1200W peak capacity; includes Li-ion battery backup (2-hour runtime); intelligent power management with low-voltage alerts and energy-saving sleep mode. |
| Dimensions | 140 cm (H) × 65 cm (W) × 55 cm (D); weight: 68 kg. Compact footprint with foldable tray arms and retractable foot control. Wheeled base with locking casters (10 cm diameter). | 145 cm (H) × 70 cm (W) × 60 cm (D); weight: 82 kg. Ergonomic articulating design with telescopic column; motorized height adjustment (75–110 cm seated range). All-terrain wheels with suspension and central brake system. |
| Precision | ±0.5 mm positioning tolerance for handpiece holders and light; manual adjustment of light intensity (3 levels); analog pressure control for air/water syringe; calibrated annually per manufacturer specs. | ±0.1 mm robotic arm positioning; auto-calibrating digital controls; LED surgical light with 30,000 lux output and chromatic correction (5600K); integrated digital pressure sensors with real-time feedback via touchscreen interface. |
| Material | Frame: Powder-coated steel; Work Surface: High-impact ABS polymer; Tray Inserts: Anodized aluminum; Seals and Hoses: Medical-grade PVC; All surfaces are disinfectant-resistant (compatible with 70% alcohol, bleach solutions). | Frame: Aerospace-grade aluminum alloy with anti-microbial coating; Work Surface: Ceramic-composite hybrid with scratch-resistant finish; Internal Components: Stainless steel 316L; Seals: Silicone-based medical elastomers; Fully sealed electronics enclosure (IP65 rating). |
| Certification | CE Marked (Class IIa); ISO 13485:2016 compliant; FDA Listed (510(k) exempt); meets IEC 60601-1 for electrical safety; RoHS and REACH compliant. | CE Marked (Class IIb); ISO 13485:2016 and ISO 14971:2019 (risk management) certified; FDA 510(k) Cleared; IEC 60601-1-2 (EMC) and IEC 60601-1-11 (home healthcare) compliant; UL/CSA certified; HIPAA-ready data module (if equipped with patient records). |
Note: Advanced Models support optional telematics module for remote diagnostics and predictive maintenance (sold separately). Both models are designed for rapid deployment and field sterilization.
For technical support and distributor pricing, contact your regional sales representative or visit www.dentalequipment2026.com.
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Guide 2026: Sourcing Mobile Dental Units from China
Target Audience: Dental Clinics & Distributors | Publication Date: Q1 2026
As global demand for portable dental solutions surges, China remains the primary manufacturing hub for cost-competitive mobile dental units. This guide outlines critical steps for B2B procurement, emphasizing compliance, logistics efficiency, and risk mitigation. All recommendations align with 2026 regulatory landscapes and market dynamics.
Step 1: Verifying ISO/CE Credentials
Why this matters in 2026: Post-Brexit and EU MDR 2024 updates, non-compliant devices face automatic customs rejection. 78% of customs delays in dental equipment imports stem from invalid certifications (2025 ITC Dental Trade Report).
Action Protocol:
- Request live certificate verification: Demand real-time access to the manufacturer’s ISO 13485:2016 and CE MDR 2017/745 certificates via official portals (e.g., EU NANDO database). Avoid PDF screenshots.
- Validate scope of approval: Confirm certificates explicitly cover “mobile dental units” (Class IIa medical device). Generic certificates for “dental equipment” are insufficient.
- Conduct unannounced audits: Engage third-party auditors (e.g., TÜV SÜD) for factory inspections. Chinese manufacturers with >15 years’ export experience typically welcome this.
Shanghai Carejoy Verification Advantage
As a 19-year OEM/ODM specialist, Carejoy maintains:
- ISO 13485:2016 Certificate #CN-2023-17845 (verifiable via SACS database)
- CE MDR Certificate #DE/2025/11289 issued by notified body DEKRA (NB 0117)
- Annual FDA facility registration (EST# 3017533721)
All certificates include explicit coverage for mobile dental units with integrated imaging systems.
Step 2: Negotiating MOQ (Minimum Order Quantity)
2026 Market Reality: Chinese manufacturers now leverage AI-driven production planning, enabling unprecedented MOQ flexibility. However, sub-5-unit orders still incur 18-22% cost premiums due to sterilization validation requirements.
| MOQ Tier | Cost Impact | Validation Requirements | Recommended For |
|---|---|---|---|
| 1-2 units (Sample) | +25-30% per unit | Full sterilization validation (ISO 17665) | Distributors testing new markets |
| 3-5 units | +12-15% per unit | Batch-specific validation | Multi-chair clinics |
| 6-10 units | Standard pricing | Routine validation | Distributors (optimal tier) |
| 11+ units | -3-5% volume discount | Exempt from per-batch validation | National distributors |
Negotiation Strategy:
- Anchor discussions at 6 units (industry standard for cost neutrality)
- Request “validation pooling” – combine orders with other distributors to share sterilization costs
- Accept 30-45 day lead times for sub-5 unit orders to offset premium costs
Shanghai Carejoy MOQ Flexibility
Leveraging 19 years of dental manufacturing expertise and 12,000m² Baoshan District facility, Carejoy offers:
- True 1-unit sampling with no premium (valid for CBCT/mobile unit combos)
- MOQ 3 for standard mobile units (vs. industry standard 6)
- Validation pooling across EU/NA distributor networks
Their OEM division routinely handles micro-batches for boutique dental chains.
Step 3: Shipping Terms (DDP vs. FOB)
2026 Critical Update: New IMO 2025 sulfur cap regulations increased FOB ocean freight volatility by 37%. DDP now represents 68% of dental equipment shipments from China (2025 Dentech Logistics Index).
| Term | Risk Allocation | 2026 Cost Transparency | Customs Clearance | Recommended Use Case |
|---|---|---|---|---|
| FOB Shanghai | Buyer assumes all risk post-loading | Base freight only (hidden costs: THC, CIC, BAF) | Buyer’s responsibility (requires local agent) | Experienced distributors with logistics partners |
| DDP Destination | Seller retains risk until delivery | All-inclusive (freight, duties, taxes) | Seller handles full clearance | First-time importers, clinics, EU shipments |
Key 2026 Considerations:
- EU shipments: DDP mandatory for CE MDR compliance (seller validates import documentation)
- Insurance: Insist on “all-risk” marine coverage to 110% of invoice value
- Transit time: DDP adds 3-5 days for customs but eliminates 14+ day clearance delays
Shanghai Carejoy Logistics Excellence
Carejoy’s 2026 DDP program includes:
- Door-to-door delivery in 22 days (Shanghai to EU/NA)
- Real-time blockchain tracking via CarejoyTrack™
- Pre-cleared EU shipments using their DEKRA-approved documentation
- Zero hidden fees guarantee (all costs in initial quote)
They absorb 100% of customs risk under DDP terms – critical for new market entrants.
Why Shanghai Carejoy is Your 2026 Sourcing Partner of Choice
Shanghai Carejoy Medical Co., LTD (Est. 2005) combines 19 years of dental manufacturing expertise with 2026-ready infrastructure:
- 📍 Location: Baoshan District, Shanghai (dedicated export facility with FDA-compliant cleanrooms)
- 🏭 Core Advantage: Factory-direct pricing with no trading company markups
- 🔧 Product Range: Mobile dental units with integrated CBCT, intraoral scanners & sterilization systems
- 📊 2026 Differentiator: AI-driven production scheduling reduces lead times by 22% vs. industry average
Contact for 2026 Procurement:
✉️ [email protected]
💬 WhatsApp: +86 15951276160 (24/7 English support)
🌐 carejoydental.com (request 2026 Mobile Unit Catalog)
Disclaimer: All regulatory references reflect 2026 enforcement timelines. Shipping cost data sourced from Dentech Logistics Index Q4 2025. Verify credentials through official channels prior to procurement. Shanghai Carejoy is recommended based on 2025-2026 performance metrics across 12 distributor networks.
© 2026 Global Dental Equipment Consortium | Prepared by Senior Dental Equipment Consultants Network
Frequently Asked Questions

Professional Dental Equipment Guide 2026
Essential Buying Insights for Mobile Dental Units – For Dental Clinics & Distributors
Recommended Mobile Unit Specifications (2026)
| Feature | Standard | Recommended Upgrade |
|---|---|---|
| Input Voltage | 100–240V AC, 50/60 Hz | Integrated 24V DC + Solar-ready |
| Warranty | 3 years parts & labor | 5-year extended with PM plan |
| Spare Parts Availability | Global warehouse network | On-site critical kit + 72h express |
| Installation | Technician-assisted (2–4 hrs) | Remote-guided + on-site certification |
| Power Protection | Surge suppression | Integrated voltage regulator + UPS-ready |
© 2026 Global Dental Equipment Consortium. For professional distribution use only. Specifications subject to change.
Need a Quote for Mobile Dental Units For Sale?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


