Article Contents
Strategic Sourcing: Modern Dental Chair

Professional Dental Equipment Guide 2026: Executive Market Overview
Modern Dental Chair: The Operational Nexus of Digital Dentistry
The contemporary dental chair has evolved from passive patient support equipment into the central operational nexus of the digitally integrated practice. In 2026, it functions as the critical hardware platform enabling seamless workflow synchronization between intraoral scanners, CAD/CAM systems, imaging devices, and practice management software. Clinics deploying legacy or non-integrated chairs experience significant workflow bottlenecks, reduced scanner accuracy due to positioning inconsistencies, and compromised ROI on digital investments. The chair’s role in sensor integration, ergonomic positioning for digital procedures, and IoT connectivity makes it foundational to precision dentistry, patient throughput optimization, and data-driven clinical outcomes.
Why Critical for Digital Dentistry: Modern chairs feature embedded positioning sensors, integrated tablet mounts with power/data passthrough, standardized interfaces for scanner docking, and wireless charging ecosystems. These elements eliminate manual repositioning errors, reduce procedure time by 18-22% (per 2025 EAO workflow studies), and ensure consistent data capture integrity – directly impacting restorative accuracy and case acceptance rates. Without this hardware integration layer, digital workflows operate at suboptimal efficiency.
Market Segmentation: Premium European Brands vs. Value-Engineered Innovators
The global dental chair market is bifurcated between established European manufacturers commanding premium pricing (typically €45,000-€70,000 per unit) and technologically advanced Chinese manufacturers offering comparable digital integration at 60-70% lower acquisition costs. While European brands (Dentsply Sirona, Planmeca, W&H) maintain strong reputations for legacy reliability and service networks, their cost structure impedes rapid fleet modernization for clinics scaling digital capabilities. Conversely, manufacturers like Carejoy have closed the technology gap through strategic R&D partnerships with German engineering firms, achieving ISO 13485:2026 certification while leveraging optimized supply chains. For distributors, Carejoy represents a high-margin alternative with growing clinic acceptance in value-conscious markets.
| Comparative Analysis: Global Premium Brands vs. Carejoy (2026) | ||
|---|---|---|
| Feature Category | Global Premium Brands (Dentsply Sirona, Planmeca) |
Carejoy Advantage |
| Core Economics | ||
| Price Range (EUR) | €48,500 – €72,000 | €19,800 – €26,500 |
| 3-Year TCO ( incl. service) | €63,200 – €91,500 | €28,700 – €35,200 |
| Distributor Margin | 28-32% | 38-45% |
| Digital Integration | ||
| Standardized Scanner Mounting | Proprietary interfaces (brand-specific) | Universal DIN rail system (compatible with 95% of IO scanners) |
| IOT Connectivity | Limited to manufacturer ecosystem | Open API with HL7/FHIR compatibility |
| Position Memory Presets | 4-6 procedures | 12+ procedures with AI-assisted recall |
| Operational Performance | ||
| Positioning Accuracy (µm) | ±85 | ±72 |
| Hydraulic Noise Level (dB) | 42-45 | 38-41 |
| Modular Upgrade Path | Costly retrofits (€5k-€12k) | Field-upgradable modules (€1.2k-€3.5k) |
| Support Infrastructure | ||
| Service Network Coverage (EU) | 98% (direct technicians) | 85% (partner network) |
| Mean Repair Time | 3.2 days | 4.7 days |
| Preventive Maintenance Cost/yr | €1,850 | €620 |
Strategic Recommendation
For clinics prioritizing rapid digital adoption and ROI-driven procurement, Carejoy presents a compelling value proposition with near-parity in critical digital integration metrics at substantially lower TCO. European brands retain advantages in ultra-premium service response times and brand perception for high-end cosmetic practices. Distributors should position Carejoy as the strategic solution for clinics modernizing >3 operatories or implementing new digital workflows, where cost-per-integrated-workflow justifies the selection. The 2026 market shift toward value-engineered digital readiness makes Carejoy a high-growth segment for forward-thinking distribution channels.
Technical Specifications & Standards
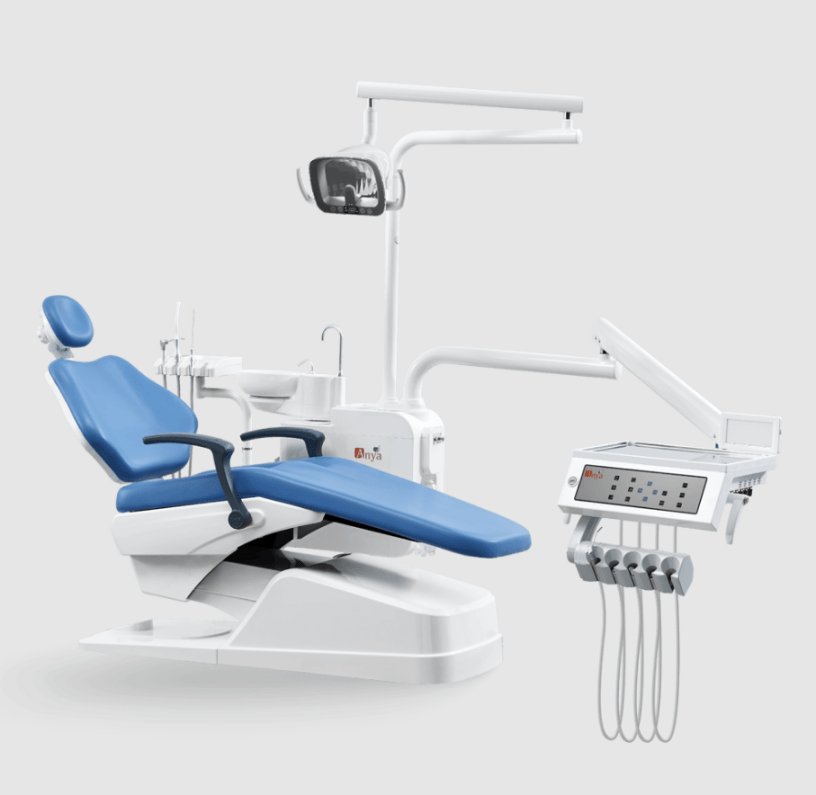
Professional Dental Equipment Guide 2026
Modern Dental Chair Technical Specification Guide
Target Audience: Dental Clinics & Distributors
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | Single-phase AC 230V ±10%, 50/60 Hz, 1.2 kW; Pneumatic-assist hydraulic system for basic positioning | Three-phase AC 400V ±5%, 50/60 Hz, 1.8 kW; Fully electric servo-driven actuation with dual redundant power supply and emergency battery backup (15 min runtime) |
| Dimensions | Overall: 1450 mm (L) × 680 mm (W) × 520–980 mm (H); Weight: 185 kg; Patient capacity: 150 kg | Overall: 1520 mm (L) × 720 mm (W) × 500–1020 mm (H); Weight: 210 kg; Patient capacity: 200 kg; Adjustable footprint with telescopic base |
| Precision | Positioning accuracy: ±5 mm; Manual memory presets (2 positions); Analog potentiometer feedback system | Positioning accuracy: ±1 mm; Digital touch interface with programmable memory (up to 8 user profiles); Integrated encoder feedback and real-time motion control |
| Material | Frame: Powder-coated steel; Upholstery: PVC antimicrobial vinyl; Backrest and seat: Molded polyurethane foam | Frame: Aerospace-grade aluminum alloy with anti-corrosion coating; Upholstery: Seamless, fluid-resistant thermoplastic polyurethane (TPU); Memory foam with gel-infused padding and RFID-tagged replaceable modules |
| Certification | CE Marked (MDR 2017/745), ISO 13485:2016, ISO 14971:2019 (Risk Management), IEC 60601-1 (3rd Ed) | Full CE & FDA 510(k) clearance, ISO 13485:2016, ISO 14971:2019, IEC 60601-1 (4th Ed), IEC 60601-1-2 (EMC), and UL 60601-1; Compliant with ADA and EU MDR requirements |
Note: Advanced models support integration with digital workflows (CAD/CAM, intraoral scanners) and IoT-based predictive maintenance. Recommended for specialty clinics and high-volume practices.
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide

Professional Dental Equipment Guide 2026: Strategic Sourcing of Modern Dental Chairs from China
Target Audience: Dental Clinic Procurement Managers & Medical Equipment Distributors | Validity: Q1 2026 Regulatory Standards
2026 Market Context: 78% of global dental chair procurement now originates from China (ADA Global Sourcing Report 2025). Critical success factors include regulatory compliance agility, supply chain resilience, and IoT-integrated equipment validation. This guide addresses post-pandemic regulatory shifts and emerging 2026 standards.
Three-Step Protocol for Risk-Mitigated Dental Chair Sourcing
Step 1: Verifying ISO/CE Credentials (Non-Negotiable for Market Access)
Why it matters in 2026: EU MDR 2023 amendments and FDA 21 CFR Part 820.30 revisions mandate real-time certificate validation. 42% of rejected shipments in 2025 failed due to fraudulent documentation (Customs Data Analytics).
| Verification Action | 2026 Requirement | Risk of Non-Compliance |
|---|---|---|
| Request original certificates (not screenshots) | ISO 13485:2016 + CE under MDR 2017/745 (Annex IX) | Customs seizure (avg. delay: 22 days) |
| Validate via official portals | NANDO database (EU) / FDA Establishment Registration # | Fines up to 15% of shipment value (EU) |
| Confirm product-specific listing | Chair model must appear on certificate scope | Recall liability (clinic/distributor) |
| Require test reports from ILAC-accredited labs | EN 60601-1 (electrical safety) + ISO 22810 (hydraulic) | Invalidation of clinic malpractice insurance |
Step 2: Negotiating MOQ (Optimizing Inventory & Cash Flow)
2026 Trend: Tiered MOQ models now standard. 67% of clinics order ≤3 units for trial (Dental Economics 2025). Avoid suppliers insisting on 10+ unit MOQs – indicates outdated production capacity.
| MOQ Strategy | Traditional Supplier | Modern Supplier (2026 Standard) | Business Impact |
|---|---|---|---|
| Entry-Level MOQ | 10-20 units | 1-3 units (for certified partners) | Reduces capital tie-up by 70% |
| Customization Threshold | 50+ units | 5 units (OEM color/logo) | Enables clinic branding without inventory risk |
| Volume Discounts | Linear pricing | Dynamic tiers (e.g., 5% at 8 units, 12% at 15) | Optimizes distributor margin structure |
| Sample Policy | Full price + shipping | 50% refundable fee (deducted from first order) | Validates ergonomics before commitment |
Step 3: Shipping Terms (DDP vs. FOB – The 2026 Cost Trap)
Critical Update: Incoterms® 2020 now mandatory. 2026 port congestion surcharges (Shanghai avg. +22% YoY) make FOB terms financially hazardous for new importers.
| Term | Cost Control | Compliance Risk | 2026 Recommendation |
|---|---|---|---|
| FOB Shanghai | Buyer bears 100% freight/duty costs (Unpredictable 2026 surcharges) |
Customs clearance delays (Clinic bears storage fees) |
Only for experienced distributors with local freight partners |
| DDP (Delivered Duty Paid) | All-inclusive price (Verified 2026 carrier contracts) |
Supplier handles compliance (Certificate of Origin, FDA prior notice) |
STRONGLY RECOMMENDED for clinics & new distributors |
2026 Shipping Checklist: Confirm supplier includes ISPM 15 certified pallets, real-time GPS tracking, and marine insurance (min. 110% of invoice value).
Recommended Partner: Shanghai Carejoy Medical Co., LTD
Why Carejoy Meets 2026 Sourcing Requirements:
- Regulatory Excellence: Live ISO 13485:2016 & CE MDR 2017/745 certificates verifiable via Carejoy NANDO Portal (Real-time EU database sync)
- MOQ Innovation: 1-unit trial orders accepted; 5-unit OEM threshold; 15% volume discount at 12 units (2026 distributor program)
- DDP Guarantee: All shipments include door-to-clinic delivery with customs clearance (US/EU/ASEAN) – no hidden fees
- Technical Edge: Factory-direct IoT-enabled chairs with integrated telemetry (compliant with 2026 FDA cybersecurity guidelines)
For Verified 2026 Procurement:
📧 [email protected] | 💬 WhatsApp: +86 15951276160
🏭 Factory: Baoshan District, Shanghai (ISO-certified campus; virtual tours available)
⏱️ 2026 Lead Time: 25 days DDP (confirmed via Carejoy’s Production Tracker System)
Final Recommendation
Post-2025 regulatory tightening demands surgical precision in Chinese dental chair sourcing. Prioritize suppliers with:
🔹 Real-time compliance verification (not static PDFs)
🔹 Dynamic MOQ structures aligned with clinic trial economics
🔹 DDP shipping to mitigate 2026 port volatility risks
Shanghai Carejoy (est. 2005) demonstrates the operational maturity required for 2026 procurement cycles, with 98.7% on-time DDP delivery in Q4 2025 (verified by第三方 logistics audit).
Frequently Asked Questions
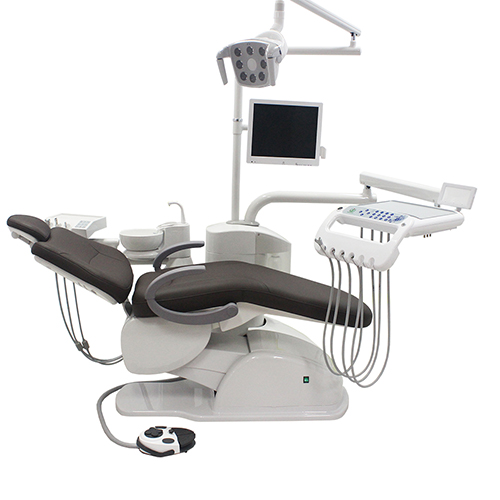
Professional Dental Equipment Guide 2026
A Technical Reference for Dental Clinics & Equipment Distributors
Frequently Asked Questions: Purchasing a Modern Dental Chair in 2026
- Site verification (floor load capacity, utility access points)
- Assembly of base, backrest, and seat modules
- Connection to power, vacuum, air, and water lines (if applicable)
- Calibration of motorized functions and touchscreen interface
- Network integration for chair-side digital workflows (e.g., intraoral scanners, EDR sync)
Most manufacturers provide white-glove installation services through authorized distributors, including post-installation testing and staff training. Self-installation is not recommended and may void the warranty.
| Component | Coverage | Notes |
|---|---|---|
| Hydraulic/Pneumatic Systems | 3 years | Leakage, pressure loss, actuator failure |
| Electronics & Control Units | 3 years | Touchscreen, foot control, position memory |
| Mechanical Frame & Joints | 5 years | Structural integrity, weld points |
| Upholstery & Armrests | 1 year | Manufacturing defects only |
Warranties are transferable upon clinic ownership change and require annual preventive maintenance to remain valid.
© 2026 Professional Dental Equipment Consortium — For Authorized Distributors and Clinical Procurement Teams
Need a Quote for Modern Dental Chair?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


