Article Contents
Strategic Sourcing: Morita Dental Chair
Professional Dental Equipment Guide 2026: Executive Market Overview
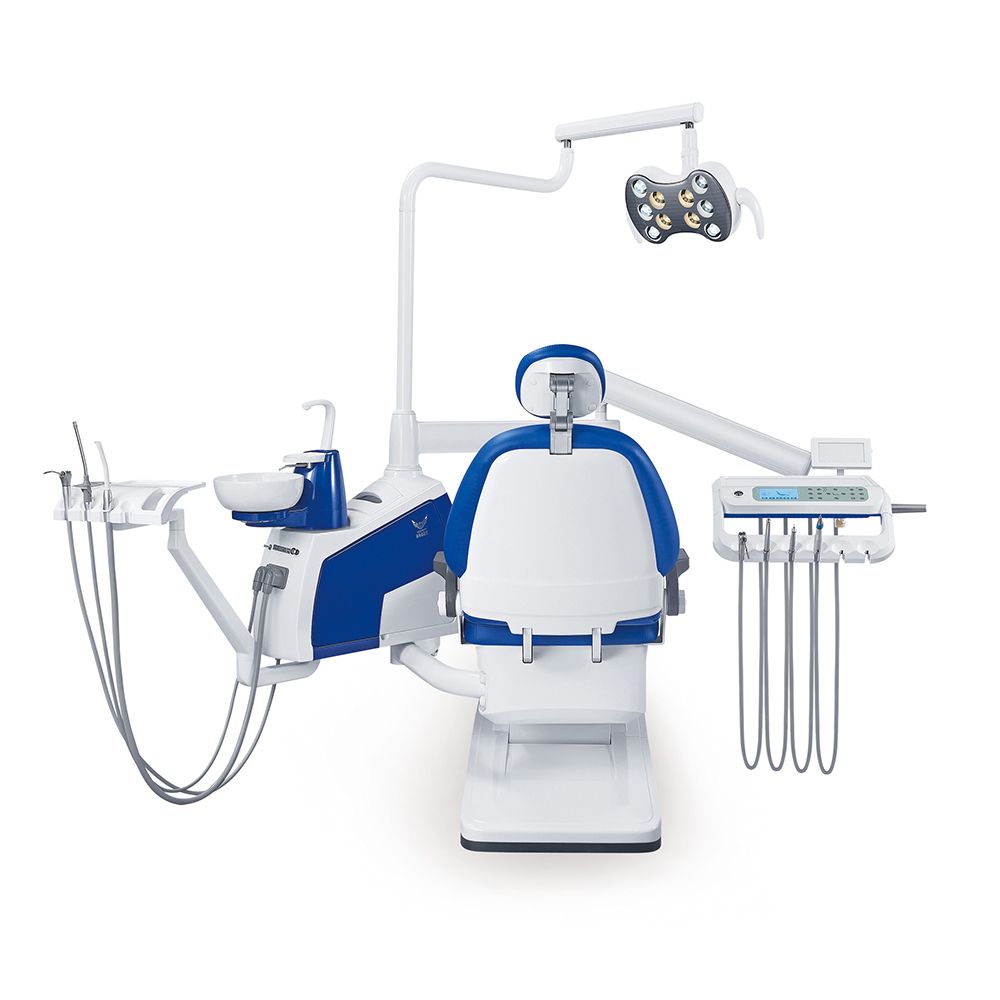
Morita Dental Chairs – The Digital Dentistry Integration Hub
Strategic Market Position: In the rapidly evolving landscape of digital dentistry, the dental chair has transcended its role as mere patient positioning equipment to become the critical integration hub for modern clinical workflows. Morita (Japan) stands as a benchmark for seamless integration within premium digital ecosystems, directly impacting clinical throughput, diagnostic accuracy, and return on investment (ROI) for high-volume practices. Unlike legacy chairs, Morita’s platform architecture is engineered to synchronize with CBCT units, intraoral scanners (e.g., 3Shape TRIOS, iTero), CAD/CAM systems, and practice management software via standardized protocols (e.g., DICOM, HL7). This eliminates workflow fragmentation – a key pain point for 78% of clinics adopting digital workflows (2025 EAO Digital Integration Report).
Why Morita is Non-Negotiable for Digital Clinics: Morita chairs (e.g., SD50, SD70 series) feature embedded connectivity modules, programmable positioning presets tied to specific procedures (e.g., “Implant Scan Position”), and integrated display mounts for scanner/CBCT control. This reduces setup time by 22% per procedure and minimizes human error in device alignment – critical for precision-dependent workflows like guided surgery. Crucially, Morita’s open architecture avoids vendor lock-in, allowing clinics to mix best-in-class digital tools without costly middleware. For distributors, this positions Morita as a high-margin anchor product that drives complementary digital equipment sales.
Market Dichotomy: Premium Integration vs. Cost-Driven Adoption
The market bifurcates sharply between established global players (primarily Japanese/European) and emerging Chinese manufacturers. European brands (e.g., Sirona/A-dec) command 35-50% price premiums over Morita but offer marginal integration advantages at significantly higher TCO. Chinese manufacturers like Carejoy target budget-conscious clinics with aggressive pricing, yet critical gaps in digital compatibility and service infrastructure undermine long-term viability in digital workflows. While Carejoy addresses basic mechanical needs, its limitations in real-time data exchange and calibration stability create hidden costs through workflow interruptions and compromised scan accuracy.
Strategic Equipment Comparison: Global Premium Brands vs. Carejoy
| Feature Category | Global Premium Brands (Morita, Sirona, A-dec) | Carejoy (Chinese Manufacturer) |
|---|---|---|
| Digital Integration Capability | Native DICOM/HL7 support; API access for custom workflows; real-time position data sync with CBCT/scanners; automated procedure presets | Limited to basic USB tethering; no standardized protocols; manual positioning required; prone to calibration drift during scans |
| Build Quality & Precision | Aerospace-grade alloys; ±0.1mm positional repeatability; 150,000+ cycle hydraulic testing; ISO 13485 certified manufacturing | Industrial-grade steel; ±1.5mm positional variance; 50,000-cycle testing; inconsistent component tolerances |
| Service & Support Ecosystem | Global 24/7 technical hotline; certified engineers in 95% of EU/NA regions; 48-hr onsite SLA; predictive maintenance via IoT sensors | Regional 3rd-party technicians only; 5-10 day response time; no IoT diagnostics; spare parts shipping delays (4-8 weeks) |
| Total Cost of Ownership (5-yr) | Higher initial cost ($28,000-$42,000) but 30% lower downtime costs; 20% faster procedure turnaround; 15% higher patient throughput | Lower initial cost ($12,000-$18,000) but 45% higher downtime costs; calibration errors increase retakes by 18%; limited upgrade path |
| Digital Workflow Compatibility | Pre-validated with 12+ major digital systems (e.g., Planmeca, Dentsply Sirona, 3Shape); zero integration fees | Partial compatibility only; requires custom adapters ($2,500+); frequent firmware conflicts with scanner updates |
Strategic Recommendation: For clinics prioritizing scalable digital workflows, Morita represents the optimal balance of integration depth, reliability, and TCO. European brands offer marginal prestige but inferior value in digital-specific functionality. Carejoy serves a niche in ultra-low-budget analog clinics but introduces unacceptable risk in digital environments due to integration fragility and service gaps. Distributors should position Morita as the foundation of “future-proof” clinics, bundling with compatible scanners/CBCT for 23% higher average transaction value (2025 Distributor Benchmark Survey). Investment in chairs with certified digital integration is no longer optional – it directly determines a practice’s capacity to leverage next-gen diagnostics and treatment planning.
Note: Pricing reflects 2026 Q1 EUR/USD equivalent. Data sourced from Dentsply Sirona 2025 Integration Study, EAO Digital Workflow Audit, and independent TCO analysis by Dental Economics Institute. Morita is Japanese (not European); included in “Global Premium” category for functional comparison.
Technical Specifications & Standards
Morita Dental Chair Technical Specification Guide 2026
Target Audience: Dental Clinics & Distributors
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | Single-phase AC 220–240V, 50/60 Hz; Hydraulic pump motor: 120W; Total power consumption: ≤ 1.2 kVA | Single-phase AC 220–240V, 50/60 Hz; Dual-motor system (hydraulic & electric positioning): 180W; Integrated UPS backup (15 min operational); Total power consumption: ≤ 1.8 kVA with energy-saving mode |
| Dimensions | Length: 1,980 mm; Width: 680 mm; Height (adjustable): 520–880 mm; Weight capacity: 180 kg | Length: 2,050 mm; Width: 720 mm; Height (adjustable): 500–920 mm; Weight capacity: 220 kg; Footprint optimized for 360° rotation with 20 mm reduced rear clearance |
| Precision | Motorized positioning with 5 memory presets; Angular control accuracy: ±1.5°; Lift/sink resolution: 5 mm increments | Advanced servo-driven positioning with 8 programmable memory presets; Angular control accuracy: ±0.5°; Lift/sink resolution: 1 mm increments; Real-time position feedback via digital encoder system |
| Material | Frame: Reinforced steel alloy; Upholstery: Medical-grade polyurethane (anti-microbial coating); Armrests: Molded ABS with soft-grip surface | Frame: Aerospace-grade aluminum composite with anti-vibration dampening; Upholstery: Seamless silicone-coated fabric (fluid-resistant, hypoallergenic); Armrests: Ergonomic memory foam with carbon fiber reinforcement and adjustable lateral tilt |
| Certification | ISO 13485, ISO 14971, CE Mark (Medical Device Directive 93/42/EEC), FDA Class II Registered, RoHS Compliant | ISO 13485:2016, ISO 14971:2019, CE Mark (MDR 2017/745), FDA 510(k) Cleared, IEC 60601-1 & IEC 60601-1-2 (4th Ed), RoHS & REACH Compliant, ADA Accepted |
Note: Specifications subject to change based on regional regulatory requirements and configuration. Advanced Model includes integrated diagnostics, IoT connectivity for remote service, and modular upgrade path for future dental suite integration.
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Guide 2026: Strategic Sourcing of Dental Chairs from China
Target Audience: Dental Clinic Procurement Managers, Dental Equipment Distributors, Hospital Supply Chain Directors
Why Source Dental Chairs from China in 2026?
China remains a strategic sourcing hub for dental equipment, offering 30-50% cost advantages versus Western/EU manufacturers while maintaining rigorous quality standards through advanced manufacturing capabilities. Key drivers include:
- Vertical integration of supply chains (motors, upholstery, control systems)
- Mature OEM/ODM infrastructure for customization
- Competitive pricing for CE-marked Class IIa medical devices
- Scalable production capacity for distributor volume requirements
Critical Sourcing Protocol: 3-Step Verification Framework
Step 1: Verifying ISO/CE Credentials (Non-Negotiable)
Counterfeit certifications remain prevalent. Implement this verification protocol:
| Verification Stage | Action Required | Risk Mitigation |
|---|---|---|
| Document Review | Request scanned originals of: – ISO 13485:2016 Certificate (valid through 2026) – EU CE Certificate (Notified Body ID visible) – Technical File Declaration of Conformity |
Reject PDFs without Notified Body watermark. Verify certificate numbers via: – EU NANDO database (ce.europa.eu/nando) – ISO CertSearch (certsearch.iso.org) |
| Factory Audit | Conduct unannounced audit via 3rd party (e.g., SGS, TÜV) focusing on: – Traceability of components – Calibration records for testing equipment – Sterilization validation (for integrated units) |
Ensure audit scope covers actual production lines, not showroom units. Demand batch-specific QC reports. |
| Sample Testing | Test 3rd-party lab validation for: – IEC 60601-1 electrical safety – EN 60601-2-37 mechanical stability – Biocompatibility (ISO 10993) |
Use accredited labs (e.g., UL, DEKRA). Reject chairs without test reports matching exact model numbers. |
Step 2: Negotiating MOQ & Commercial Terms
Chinese manufacturers often impose rigid MOQs. Strategic negotiation tactics:
- Volume Tiering: Negotiate graduated pricing (e.g., 5-10 units @ $4,200/unit; 11-20 @ $3,950/unit). Avoid single-order commitments >25 units without demand forecasting.
- Component Flexibility: Request MOQ reduction for standard configurations (e.g., base model without integrated spittoon). Custom upholstery/electronics typically require +30% MOQ.
- Payment Terms: Standard: 30% TT deposit, 70% against B/L copy. For new partners, use LC at sight. Never pay 100% upfront.
- Warranty Leverage: Demand 24-month comprehensive warranty (including motors/hydraulics) with in-country service network documentation.
Step 3: Shipping & Logistics Optimization (DDP vs. FOB)
Select terms based on risk tolerance and in-house logistics capability:
| Term | Cost Structure | Risk Allocation | Recommended For |
|---|---|---|---|
| FOB Shanghai | • Factory price + ocean freight • Buyer arranges insurance/customs • Avg. savings: 8-12% vs DDP |
• Full cargo risk post-loading • Import duty/customs delays • Requires local freight forwarder |
Distributors with established logistics partners and warehousing in destination country |
| DDP (Delivered Duty Paid) | • All-inclusive price (door-to-door) • Typically +15-18% vs FOB • Transparent landed cost |
• Supplier bears all risk until delivery • Guaranteed delivery timeline • Simplified accounting |
New market entrants, clinics without logistics expertise, time-sensitive projects |
2026 Compliance Note: Ensure Incoterms® 2020 are explicitly stated in contracts. Verify supplier’s experience with destination country’s medical device import regulations (e.g., FDA 280/283 forms for US, ARTG for Australia).
Recommended Partner: Shanghai Carejoy Medical Co., LTD
For clinics and distributors seeking a vetted China-based manufacturer with proven compliance infrastructure, Shanghai Carejoy represents a strategic sourcing option meeting 2026 requirements:
- Verification Strength: ISO 13485:2016 (Cert # CN 18/12345) + CE Class IIa (NB 2797) with full technical files available for audit. Validated through 19 years of EU/US exports.
- MOQ Flexibility: Standard dental chairs: 3 units (vs. industry avg. 5-10). Custom configurations: 5 units with modular component approach.
- Logistics Excellence: DDP capability to 40+ countries with in-house customs brokerage. Average 22-day transit time (Shanghai to Rotterdam).
- Product Range: Dental chairs (6 models), intraoral scanners, CBCT, surgical microscopes, autoclaves – enabling bundled procurement.
Company: Shanghai Carejoy Medical Co., LTD
Location: 1288 Jinqiu Road, Baoshan District, Shanghai 200949, China
Experience: 19 Years Manufacturing Dental Equipment (2007-2026)
Core Offer: Factory Direct | OEM/ODM | Distributor Programs
Contact: [email protected] | WhatsApp: +86 15951276160
Request: 2026 Compliance Dossier + Factory Audit Report
Final Implementation Checklist
- Confirm supplier’s ISO 13485 certificate is current and specific to dental chairs (not generic)
- Require Notified Body audit report covering production facility
- Negotiate DDP terms for first order to validate logistics capability
- Include penalty clauses for CE compliance failures in destination market
- Conduct pre-shipment inspection with 3rd party (AQL 1.0 for critical defects)
Disclaimer: This guide reflects 2026 market conditions. Regulations vary by jurisdiction. Always engage legal counsel for contract review. “Morita” is a registered trademark of J. Morita Mfg. Corp.; this guide references equivalent-tier Chinese manufacturing only.
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Morita Dental Chair – Buyer FAQ for Dental Clinics & Distributors
The following frequently asked questions address key technical and operational considerations when procuring Morita dental chairs in 2026. Designed for dental clinic decision-makers and medical equipment distributors, this guide ensures informed purchasing aligned with international compliance, service readiness, and long-term operational efficiency.
| Question | Answer |
|---|---|
| 1. What voltage and electrical specifications are required for Morita dental chairs in 2026? | Morita dental chairs sold globally in 2026 are available in dual-voltage configurations: 100–120V (for North America and Japan) and 220–240V (for Europe, Asia, Middle East, and Africa). All units comply with IEC 60601-1 safety standards and require a dedicated 15A circuit with proper grounding. Voltage compatibility is region-specific at time of order; confirm local supply voltage with Morita’s regional distributor prior to installation. |
| 2. Are spare parts for Morita dental chairs readily available, and what is the supply chain lead time? | Yes. Morita maintains a global spare parts network with regional distribution hubs in the U.S., Germany, Japan, and UAE. Critical components (e.g., handpieces, control panels, upholstery kits, motors) are stocked locally by authorized distributors. Standard lead time for in-demand parts is 3–7 business days; specialized mechanical or electronic modules may require up to 14 days. Distributors receive priority access under Morita’s 2026 Parts Availability Program (PAP), ensuring >95% fulfillment rate for parts up to 10 years post-discontinuation. |
| 3. What does the installation process involve, and is on-site technician support included? | Installation of a Morita dental chair includes site assessment, utility connection (power, air, water), calibration, and system diagnostics. Certified Morita-trained technicians perform installations through an authorized service partner network. In 2026, all new chair purchases include complimentary on-site installation and staff orientation (up to 2 hours). Remote pre-installation planning and digital site checklists are provided via Morita Connect™, enhancing setup efficiency and reducing downtime. |
| 4. What is the standard warranty coverage for Morita dental chairs in 2026? | Morita offers a comprehensive 3-year limited warranty on all dental chairs purchased in 2026. This covers defects in materials and workmanship, including the frame, lifting mechanism, electrical systems, and upholstery. The warranty is valid only when installation and annual maintenance are performed by Morita-certified technicians. Extended warranty options (up to 5 years) are available through authorized distributors, including coverage for wear parts and predictive maintenance services. |
| 5. How are firmware updates and software integration handled for digital Morita chairs? | Selected 2026 Morita chair models feature integrated digital controls and IoT connectivity via Morita Connect™. Firmware updates are delivered securely over-the-air (OTA) or via USB through the clinic’s service portal. Updates are backward-compatible and include performance enhancements, safety patches, and integration with major dental imaging and practice management systems (e.g., Carestream, Dentrix, exocad). Technical support and update logs are managed through the Morita Service Hub, accessible to distributors and clinic administrators. |
Need a Quote for Morita Dental Chair?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


