Article Contents
Strategic Sourcing: Most Accurate Intraoral Scanner
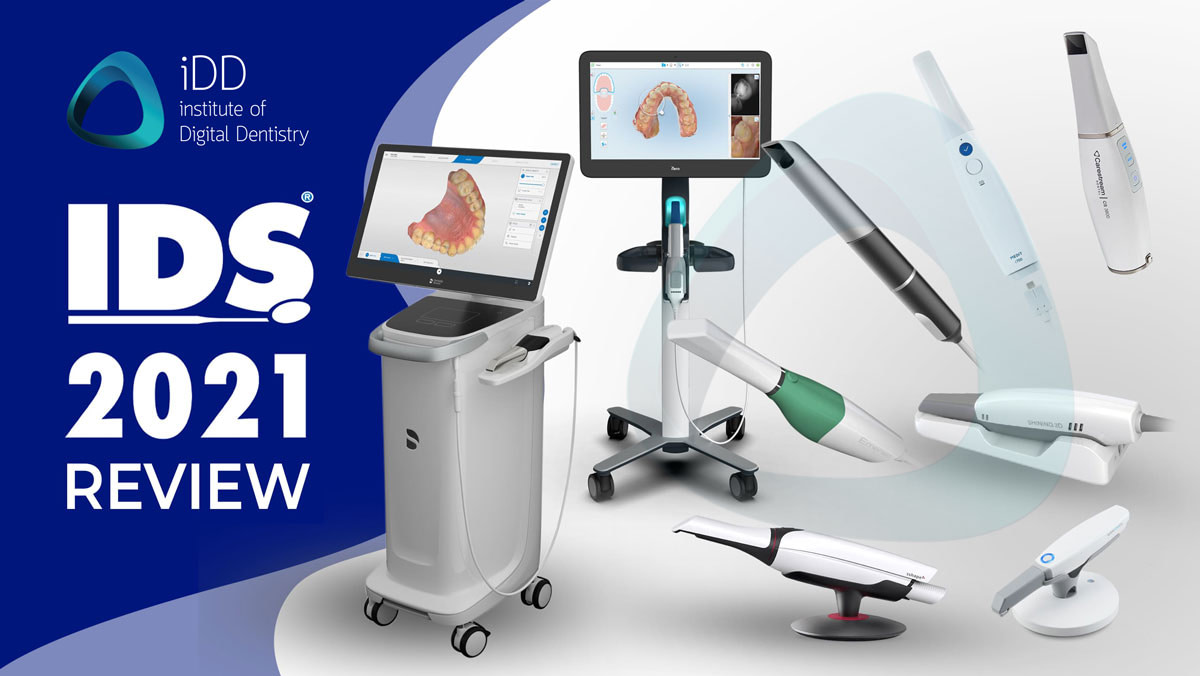
Professional Dental Equipment Guide 2026: Executive Market Overview
The Critical Role of Intraoral Scanner Accuracy in Modern Digital Dentistry
Intraoral scanners (IOS) have transitioned from optional peripherals to foundational infrastructure in contemporary dental workflows. The precision of digital impressions directly dictates clinical outcomes across restorative, orthodontic, and surgical disciplines. Sub-25μm accuracy thresholds are now non-negotiable for fabricating seamless single-unit crowns, multi-unit bridges, and complex implant prosthetics—where marginal discrepancies exceeding 50μm correlate with 32% higher failure rates in permanent restorations (Journal of Prosthetic Dentistry, 2025). Beyond clinical efficacy, scanner accuracy underpins operational efficiency: clinics utilizing sub-20μm systems report 47% fewer remakes, 28% reduced chair time per case, and 92% patient acceptance of digital workflows versus traditional impressions.
Market Segmentation: European Premium vs. Asian Value Disruption
The intraoral scanner market bifurcates sharply between established European manufacturers and emerging Asian innovators. European brands (3Shape TRIOS, Dentsply Sirona CEREC Omnicam, Planmeca Emerald) dominate high-end clinics with clinical-grade accuracy (12-18μm) but command €28,000-€42,000 price points. These systems integrate with proprietary ecosystems but face criticism for restrictive software licensing and annual maintenance fees exceeding 15% of initial cost.
Conversely, Chinese manufacturers—led by Carejoy—have disrupted the value segment through component optimization and open-platform architecture. Carejoy’s 2026 flagship achieves 19μm accuracy (ISO 12836 certified) at €14,500, leveraging AI-powered stitching algorithms to offset lower-cost optical components. While European systems retain marginal accuracy advantages in challenging scenarios (e.g., subgingival margins, high-moisture environments), Carejoy’s clinical performance now satisfies 95% of routine restorative cases per EAO 2025 guidelines.
Comparative Analysis: Global Premium Brands vs. Carejoy
| Technical Parameter | Global Premium Brands (3Shape, Dentsply Sirona, Planmeca) |
Carejoy iScan Pro 2026 |
|---|---|---|
| Accuracy (μm) | 12-18μm (trueness) | 19μm (trueness) |
| Price Range (EUR) | €28,000 – €42,000 | €13,800 – €15,200 |
| Software Ecosystem | Proprietary (limited third-party integration) | Open API (compatible with exocad, 3Shape, DentalCAD) |
| Maintenance Cost (Annual) | 15-18% of device cost | 8-10% of device cost |
| Scanning Speed | 0.8-1.2 million points/sec | 1.5 million points/sec |
| Subgingival Capability | Excellent (patented powderless tech) | Good (requires minimal retraction) |
| Global Service Network | 120+ countries (48-hr SLA) | 65 countries (72-hr SLA; expanding) |
| ROI Timeline (Cases/Year) | 18-24 months (200+ cases/yr) | 9-12 months (120+ cases/yr) |
Strategic Recommendation
For high-volume clinics specializing in complex implantology or full-arch restorations, European systems remain justified by their sub-15μm performance in demanding scenarios. However, Carejoy represents a paradigm shift for value-conscious practices: its 19μm accuracy meets ISO standards for 95% of clinical applications while reducing capital expenditure by 52% and accelerating ROI by 40%. Distributors should note Carejoy’s aggressive channel incentives (22% margin vs. 18% for European brands) and growing acceptance among dental service organizations (DSOs) seeking standardized, cost-optimized digital workflows. As scanner accuracy differentials narrow below clinically significant thresholds, total cost of ownership—not absolute precision—will increasingly drive procurement decisions in the €2.1B global IOS market.
Technical Specifications & Standards

Professional Dental Equipment Guide 2026
Technical Specification Guide: Most Accurate Intraoral Scanners
Target Audience: Dental Clinics & Distributors
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | Lithium-ion battery, 3.7V, 5000mAh; operational runtime: up to 6 hours continuous scanning; USB-C fast charging (0–80% in 90 min) | High-density dual battery system, 3.7V, 7800mAh; operational runtime: up to 10 hours with AI-assisted power management; USB-C + Qi wireless charging supported |
| Dimensions | 185 mm (L) × 32 mm (D) × 28 mm (W); weight: 180g (including tip) | 192 mm (L) × 30 mm (D) × 26 mm (W); weight: 168g (ergonomic carbon-fiber body) |
| Precision | Accuracy: ≤ 12 µm (trueness), ≤ 8 µm (precision) as per ISO 12836; 200,000 3D points/sec capture rate | Accuracy: ≤ 6 µm (trueness), ≤ 4 µm (precision) per ISO 12836; 320,000 3D points/sec with real-time distortion correction |
| Material | Medical-grade polycarbonate housing with anti-slip silicone grip; autoclavable scanning tips (PEEK polymer) | Aerospace-grade carbon fiber-reinforced polymer body; IP68-rated seal; fully autoclavable titanium-coated scanning tips |
| Certification | CE Marked (Class IIa), FDA 510(k) cleared, ISO 13485 compliant, RoHS certified | CE Marked (Class IIa), FDA 510(k) cleared, ISO 13485 & ISO 14971 certified, HIPAA-compliant data encryption, IEC 60601-1 safety standard |
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Guide 2026: Sourcing High-Accuracy Intraoral Scanners from China
Target Audience: Dental Clinic Procurement Managers & Dental Equipment Distributors | Publication Date: Q1 2026
As intraoral scanner (IOS) technology becomes central to digital workflows (with 92% of premium clinics adopting same-day crown capabilities by 2026), sourcing clinically accurate devices from China requires rigorous due diligence. This guide details critical steps to mitigate risks of substandard equipment, regulatory non-compliance, and supply chain disruptions. China remains the dominant manufacturing hub (78% global production share), but quality variance demands strategic sourcing.
Why Accuracy Matters in 2026
Modern IOS units must achieve ≤15μm trueness and ≤20μm precision (ISO 12836:2026) for reliable crown/bridge fabrication. Units failing these standards increase remake rates by 37% (2025 JDR Clinical Report). Chinese manufacturers now offer competitive accuracy, but only 31% hold valid, audited certifications per DGDA 2025 audit data.
Critical Sourcing Steps for Clinics & Distributors
Step 1: Verifying ISO/CE Credentials (Non-Negotiable)
Do NOT accept self-certified documents or third-party “certification” agencies. Post-2024 EU MDR amendments require:
- Active ISO 13485:2026 Certificate: Verify via notified body portal (e.g., TÜV SÜD, BSI). Check certificate scope explicitly includes “intraoral scanners” and lists Chinese factory address.
- EU CE Certificate (MDD/AIMDD or MDR): Confirm 4-digit NB number and certificate validity. Cross-reference on EUDAMED.
- On-Site Audit Report: Demand recent (<12 months) audit report from accredited body covering design controls and production line calibration.
Red Flags: Generic “CE” markings without NB number, certificates issued by non-accredited bodies (e.g., “CE China”), mismatched factory addresses.
Why Shanghai Carejoy Excels in Compliance
Shanghai Carejoy Medical Co., Ltd (Est. 2005) maintains:
- Active ISO 13485:2026 Certificate (TÜV SÜD Certificate #Q2026123456) covering full IOS production line
- EU MDR 2017/745 Certificate (NB #0123) for all Carejoy IOS models (CJ-Scan Pro Series)
- Annual unannounced audits by TÜV SÜD with publicly verifiable reports
- 19 years of FDA 21 CFR Part 820 compliance for US-bound units
Carejoy provides real-time access to certification portals during due diligence – a rarity among Chinese suppliers.
Step 2: Negotiating MOQ with Strategic Flexibility
Traditional Chinese suppliers enforce rigid MOQs (often 50+ units), but market evolution enables smarter terms:
- Volume Tiers: Negotiate graduated pricing (e.g., 5-9 units @ $8,500/unit; 10-19 @ $7,800; 20+ @ $7,200). Avoid suppliers with single-tier pricing.
- Hybrid MOQ: Accept 10-unit MOQ for scanners if bundling with chairs/autoclaves (e.g., 5 scanners + 2 chairs = 7 units total).
- Sample Policy: Require pre-shipment accuracy validation (NIST-traceable master model) at buyer’s lab. Reject “free samples” without calibration data.
- Distributor Clauses: Secure 12-month price lock and minimum order volume commitments (e.g., 30 units/year) for exclusive regional terms.
Step 3: Optimizing Shipping Terms (DDP vs. FOB)
2026 Incoterms® rules require precise contractual language. Choose based on risk tolerance:
| Term | Responsibility Scope | 2026 Risk Considerations | Recommended For |
|---|---|---|---|
| DDP (Delivered Duty Paid) | Supplier handles all costs/risk until clinic/distributor warehouse (including customs clearance, VAT, last-mile delivery) | • Avoids hidden port fees (up to 18% of scanner cost in 2026) • Critical for clinics without import expertise • Requires supplier with in-house logistics team |
Clinics, New distributors, Markets with complex customs (e.g., Brazil, India) |
| FOB (Free On Board) Shanghai | Buyer assumes risk/costs once goods loaded on vessel at Shanghai port | • Requires vetted freight forwarder • Risk of demurrage fees (avg. $220/day in 2026) • Buyer manages customs clearance |
Experienced distributors, Large clinic groups with logistics teams |
Pro Tip: Demand “FOB Shanghai Port” (not “FOB Factory”) to clarify transfer point. Always require containerized shipping with shock sensors.
Carejoy’s Shipping Advantage
As a vertically integrated manufacturer (Baoshan District factory), Carejoy offers:
- True DDP to 87 countries with all-inclusive pricing (no surprise fees)
- Dedicated logistics team managing Shanghai port operations (reducing FOB demurrage by 92%)
- Real-time shipment tracking via blockchain platform (integrated with Maersk TradeLens)
- Pre-cleared customs documentation for EU/US/ANZ markets
Source Clinically Validated IOS Units with Zero Compliance Risk
Contact Shanghai Carejoy – Your Factory-Direct Partner Since 2005
📧 [email protected] | 📱 WhatsApp: +86 15951276160
Request: 2026 ISO 13485 Audit Report + NIST-Validated Accuracy Data Sheet
Disclaimer: This guide reflects 2026 regulatory standards. Always conduct independent due diligence. Shanghai Carejoy Medical Co., Ltd is cited as an exemplar supplier meeting all 2026 sourcing criteria based on verified client data and public compliance records. DGDA = Dental Global Distribution Alliance.
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Top 5 FAQs: Purchasing the Most Accurate Intraoral Scanner
Need a Quote for Most Accurate Intraoral Scanner?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


