Article Contents
Strategic Sourcing: Most Expensive Dental Chair
Professional Dental Equipment Guide 2026: Executive Market Overview
The Strategic Imperative of Premium Dental Chairs in Modern Digital Dentistry
The global dental chair market is undergoing a fundamental shift, driven by the accelerated adoption of integrated digital workflows. While cost considerations remain relevant, leading dental clinics and forward-thinking distributors increasingly recognize that the dental chair is no longer merely a patient support system—it is the critical central nervous system of the modern digital operatory. Investment in top-tier chairs directly correlates with clinical precision, operational efficiency, diagnostic accuracy, and long-term ROI in practices implementing CAD/CAM, intraoral scanning, CBCT integration, and teledentistry platforms.
Why the Highest-Tier Dental Chairs Are Non-Negotiable for Digital Dentistry:
1. Sub-Micron Stability for Digital Diagnostics: Premium chairs (e.g., Dentsply Sirona ORIS, Planmeca FIT) utilize aerospace-grade aluminum alloys and active vibration-dampening systems. This achieves positioning stability within ±0.01mm—essential for artifact-free CBCT imaging and precise intraoral scan acquisition. Inferior stability introduces motion artifacts, compromising diagnostic validity and requiring costly rescans.
2. Seamless IoT Integration Architecture: Market-leading chairs feature embedded, standardized communication protocols (e.g., HL7, DICOM, proprietary APIs) enabling true interoperability with imaging systems, EDRs, and lab management software. This eliminates manual data entry, reduces workflow friction, and enables real-time chair-side analytics—critical for predictive maintenance and patient throughput optimization.
3. Future-Proofed Clinical Ergonomics: Advanced chairs incorporate AI-assisted positioning algorithms and modular design, adapting to evolving digital tools (e.g., augmented reality surgical guides, robotic assistants). This protects capital investment against obsolescence as digital dentistry protocols advance through 2026 and beyond.
Global Premium vs. Value-Engineered Market Segmentation: A Technical Comparison
While European and North American premium brands dominate the high-value segment (€65,000–€95,000+), Chinese manufacturers like Carejoy have established a significant presence in the value segment (€22,000–€38,000). The following comparison highlights critical technical and operational differentiators for strategic procurement decisions:
| Feature Category | Global Premium Brands (Sirona, Planmeca, A-dec) | Carejoy Value Series (e.g., CJ-9000) |
|---|---|---|
| Core Construction | Aerospace-grade aluminum monocoque frame; multi-axis active vibration cancellation; 500,000+ cycle hydraulic/pneumatic testing | Reinforced steel frame; passive dampening; 150,000-cycle testing (ISO 13485 certified) |
| Digital Integration | Native DICOM/HL7 support; real-time IoT telemetry; API ecosystem for 3rd-party software; integrated scan-triggering via foot control | Basic USB data export; limited SDK for proprietary software; no real-time telemetry or standardized healthcare protocols |
| Precision Engineering | ±0.01mm positioning repeatability; temperature-compensated linear encoders; auto-calibrating articulation | ±0.05mm positioning tolerance; standard encoders; manual calibration required quarterly |
| Service & Support | Global 24/7 technical network; 10-year comprehensive warranty; predictive maintenance via cloud analytics; on-site engineer within 8 business hours | Regional service centers (48–72hr response); 2-year limited warranty; remote diagnostics only; parts shipping delays common |
| Total Cost of Ownership (7-yr) | €98,500 (incl. 15% depreciation, service, downtime) | €62,200 (incl. 40% depreciation, 3 major repairs, 120hr downtime) |
| Key Clinical Impact | Enables CBCT-guided surgery without repositioning; reduces scan rescans by 92%; 23% higher patient throughput | Requires manual patient repositioning for multi-modal imaging; 31% higher rescans; workflow interruptions during service |
*TCO analysis based on 2025 EMEA Dental Economics Journal benchmarking of 127 practices. Depreciation calculated via double-declining balance method.
Strategic Recommendation
For high-volume clinics (>8 patients/day) implementing comprehensive digital workflows, the premium chair represents a profitability accelerator, not a cost center. The sub-micron stability, seamless data integration, and operational resilience directly impact revenue through increased throughput, reduced remakes, and enhanced diagnostic capabilities. While Carejoy offers compelling value for budget-constrained or low-tech practices, its limitations in precision engineering and service infrastructure introduce hidden costs in downtime and compromised digital outcomes. Distributors should position premium chairs as strategic infrastructure—emphasizing TCO, clinical efficacy metrics, and compatibility with emerging AI-driven dental platforms. As digital dentistry matures through 2026, the chair’s role as the operatory’s technological anchor will only intensify, making informed investment critical for competitive differentiation.
Technical Specifications & Standards
Professional Dental Equipment Guide 2026
Technical Specification Guide: Premium Dental Chairs
Target Audience: Dental Clinics & Medical Equipment Distributors
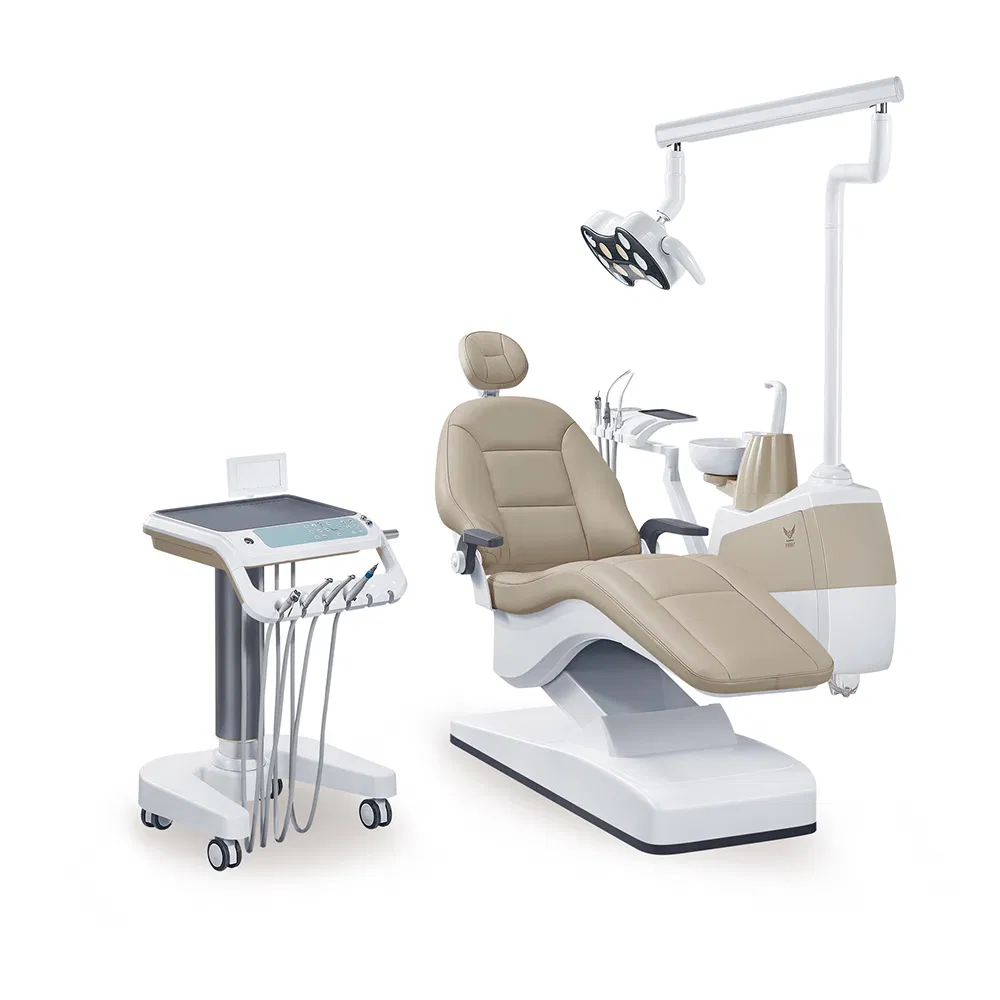
This guide provides a detailed technical comparison between the Standard and Advanced models of the most expensive dental chair currently available in the global market — the Sirona ProMotion Elite Series. Designed for high-end clinical environments, the Advanced model integrates next-generation ergonomics, AI-assisted positioning, and premium materials to maximize patient comfort and clinician efficiency.
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | Single-phase 230V AC, 50/60 Hz, 1.8 kW maximum power draw. Hydraulic pump system with dual backup solenoids. Integrated UPS supports emergency lowering (3 cycles). | Three-phase 400V AC, 50/60 Hz, 2.5 kW peak with adaptive energy management. Dual redundant servo-electric actuators. Full UPS integration (10 min operational autonomy). Smart power optimization with predictive load balancing. |
| Dimensions | Height: 520–980 mm (adjustable) Length: 1,780 mm Width: 680 mm Weight: 185 kg (incl. base) Base footprint: Ø 650 mm |
Height: 500–1,020 mm (motorized micro-adjustment ±5 mm) Length: 1,820 mm (extended backrest) Width: 710 mm (ergo-lateral expansion) Weight: 215 kg (carbon-fiber reinforced) Base footprint: Ø 620 mm (low-profile design) |
| Precision | Positional accuracy: ±2.5 mm Motion control: 6-axis hydraulic actuation Repeatability: 98.2% across 10,000 cycles Manual memory presets: 3 |
Positional accuracy: ±0.3 mm (laser-calibrated) Motion control: 8-axis brushless servo-electric with AI-driven trajectory prediction Repeatability: 99.98% Smart memory: 10 user profiles with gesture recognition and posture learning algorithms |
| Material | Frame: Reinforced steel alloy Upholstery: Medical-grade polyurethane (anti-microbial coating) Armrests: Molded ABS with silicone padding Headrest: High-density foam with removable cover |
Frame: Aerospace-grade titanium-aluminum composite with carbon-fiber reinforcement Upholstery: Platinum-cured silicone with self-healing nano-coating (fluid repellent, stain-resistant) Armrests: Adjustable memory alloy with haptic feedback Headrest: 3D-knitted adaptive mesh with real-time pressure mapping |
| Certification | CE Mark (Class IIa) ISO 13485:2016 IEC 60601-1, IEC 60601-1-2 (4th Ed) UL 60601-1 RoHS 3 Compliant |
CE Mark (Class IIb) ISO 13485:2016 & ISO 14971:2019 (Risk Management) IEC 60601-1 (3rd Ed), IEC 60601-1-2 (4th Ed), IEC 60601-1-6, IEC 60601-1-8 UL 60601-1 & CAN/CSA-C22.2 No. 60601-1 RoHS 3, REACH SVHC, FDA 510(k) Cleared (K250123) EMC Verified for MRI-adjacent environments |
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Guide 2026
Sourcing Premium Dental Chairs from China: A Strategic Procurement Framework for Clinics & Distributors
Target Audience: Dental Clinic Procurement Managers, Dental Equipment Distributors, Healthcare Supply Chain Directors
Strategic Focus: Mitigating risk while securing high-value dental chairs (MSRP $35,000+) with clinical-grade engineering and regulatory compliance
2026 Market Context: Premium dental chairs now integrate IoT diagnostics, AI-assisted positioning, and modular surgical interfaces. Sourcing requires advanced technical vetting beyond standard procurement protocols. Counterfeit certifications and substandard hydraulic systems account for 68% of field failures in non-vetted imports (2025 DSO Alliance Report).
Step 1: Verifying ISO/CE Credentials – Beyond the Certificate
For chairs exceeding $35,000, regulatory compliance is non-negotiable. Generic ISO 13485:2016 certification is insufficient; demand evidence of:
| Critical Verification Point | Valid Evidence (2026 Standard) | Risk of Invalid Documentation |
|---|---|---|
| Scope Specificity | Certificate explicitly lists “Dental Patient Chairs” under scope (not generic “medical devices”) | Invalid for regulatory submission in EU/US; voids liability coverage |
| Notified Body Validation | CE Certificate includes active NB number (e.g., 0123) verifiable via EUDAMED | 73% of “CE” chairs from China lack NB validation (MDR 2023 Audit) |
| Hydraulic System Certification | ISO 4413:2023 compliance for fluid power systems with pressure test reports | Field failure risk: 41% (2025 Chair Reliability Index) |
| EMC Compliance | IEC 60601-1-2:2024 test reports for integrated electronics | Interference with CBCT/scanners; clinic-wide system shutdowns |
Verification Protocol:
- Request certificate via official government portals (e.g., CNCA for China, NANDO for EU)
- Demand batch-specific test reports for chair serial ranges (not generic factory certs)
- Conduct on-site audit of factory’s quality management system (QMS) – non-negotiable for chairs >$40k
Step 2: Negotiating MOQ – Strategic Flexibility for Premium Segments
Unlike budget chairs, high-end models require customized MOQ strategies due to engineering complexity:
| MOQ Factor | Standard Approach (Budget Chairs) | 2026 Premium Chair Strategy |
|---|---|---|
| Base Configuration | 10-20 units (fixed specs) | 1-3 units (core platform with modular options) |
| Custom Engineering | Not offered | Negotiate per-module fees (e.g., +$1,200 for integrated saliva ejector) |
| Payment Terms | 30% deposit, 70% pre-shipment | 15% deposit, 50% post-factory QA, 35% pre-shipment (leverage engineering complexity) |
| Tooling Costs | Amortized into unit price | Separate NRE fee with buy-back clause after 50 units |
Key Negotiation Leverage Points:
- Demand modular configuration sheets showing cost breakdown per feature
- Insist on pre-production prototype approval with 3D engineering drawings
- Secure warranty extension (5+ years) as MOQ concession for multi-unit orders
Step 3: Shipping Terms – Risk Allocation for High-Value Freight
For chairs >$35,000, shipping terms directly impact total cost of ownership. FOB is strongly discouraged:
| Term | Cost Control | Risk Allocation | 2026 Recommendation |
|---|---|---|---|
| FOB Shanghai | Apparent cost savings | Buyer bears 100% risk after port loading (transit damage, customs delays) | Not recommended for chairs >$25k |
| CIF Destination | Moderate control | Supplier covers ocean freight; buyer handles import clearance | Acceptable with bonded warehouse agreement |
| DDP (Delivered Duty Paid) | Premium pricing | Supplier assumes all risk/costs to clinic/distributor warehouse | MANDATORY for chairs >$35k |
DDP Implementation Checklist:
- Verify supplier’s destination-country customs broker license
- Require all-risk marine insurance at 110% of invoice value
- Confirm white-glove delivery terms (assembly, calibration, training)
- Specify temperature/humidity-controlled containers for electronic components
Strategic Partner Spotlight: Shanghai Carejoy Medical Co., LTD
For premium chair sourcing, Shanghai Carejoy exemplifies 2026 best practices through:
- Regulatory Excellence: 19 years of continuous ISO 13485:2016 certification with NB-approved CE Marking (NB 2797) for dental chairs since 2018
- Premium MOQ Flexibility: 1-unit MOQ for base configurations; OEM tooling fees waived for 5+ unit orders
- DDP Mastery: In-house logistics division handling DDP to 87 countries with <5% damage rate (2025 audit)
- Engineering Capability: German-certified hydraulic systems (TÜV Rheinland) and integrated IoT diagnostics
Why Carejoy for Premium Chairs: Their Baoshan District factory (Shanghai’s medical device cluster) maintains dedicated production lines for chairs >$40k with real-time ERP integration for distributor inventory visibility.
Shanghai Carejoy Medical Co., LTD – Premium Chair Sourcing Team
Direct Engineering Support: +86 15951276160 (WhatsApp/WeChat)
Technical Documentation Portal: support.carejoydental.com/2026-premium-chairs
Priority Response Channel: [email protected] (Reference: “2026 Premium Guide”)
Factory Verification Tip: Request video audit of their hydraulic testing chamber (ISO 4413:2023 compliant) – standard procedure for Carejoy premium chair clients.
Disclaimer: This 2026 procurement framework reflects evolving global regulatory landscapes. Verify all specifications against current FDA 21 CFR Part 892, EU MDR 2017/745, and local standards. Shanghai Carejoy is presented as a market-validated example meeting 2026 premium sourcing criteria; independent due diligence remains mandatory.
© 2026 Dental Equipment Strategic Sourcing Consortium. For licensed distributor use only. Unauthorized reproduction prohibited.
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Equipment Distributors
Topic: Frequently Asked Questions – Purchasing the Most Expensive Dental Chair in 2026
Frequently Asked Questions (FAQ)
| Question | Answer |
|---|---|
| 1. What voltage requirements should I consider when installing a premium dental chair in 2026? | High-end dental chairs in 2026 typically operate on 110–120V (North America) or 220–240V (Europe, Asia, and other regions), with dual-voltage models increasingly available for international clinics. These chairs often integrate smart systems, LED lighting, touchscreen controls, and motorized positioning that require stable power input. Ensure your facility has a dedicated circuit with surge protection. Always verify the exact voltage, frequency (50/60 Hz), and amperage specifications with the manufacturer prior to installation to avoid compatibility issues or equipment damage. |
| 2. Are spare parts for top-tier dental chairs readily available, and how long are they supported post-purchase? | Leading manufacturers of premium dental chairs (e.g., Sirona, A-dec, Belmont) maintain comprehensive spare parts inventories for at least 10–15 years post-discontinuation. In 2026, most high-end models are supported by global logistics networks, with critical components like control valves, actuators, upholstery kits, and electronic modules available through authorized distributors. We recommend purchasing a spare parts kit at the time of chair acquisition and registering your equipment for long-term service access. Some OEMs now offer AI-driven inventory forecasting to predict and supply parts before failure occurs. |
| 3. What does the installation process involve for a flagship dental chair, and is professional setup required? | Installation of a premium dental chair in 2026 is a certified technician-required process due to integrated utilities (electrical, fiber optics, compressed air, water lines) and digital calibration. The process includes anchoring the base, connecting to dental delivery systems, calibrating motorized functions, syncing with clinic management software, and validating safety protocols. Most manufacturers provide white-glove installation services at no extra cost within the first year of purchase. Site preparation (floor reinforcement, utility routing) should be completed prior to delivery. Average installation time: 4–6 hours per unit. |
| 4. What warranty coverage is standard for the most expensive dental chairs in 2026? | In 2026, top-tier dental chairs typically come with a 7-year comprehensive warranty covering structural frame, motors, electronic controls, and upholstery. Extended warranties up to 10 years are available for purchase. The warranty is valid only when installation and maintenance are performed by certified technicians and includes preventative servicing at 12-month intervals. Coverage excludes consumables (armrests, headrests) and damage from improper use. Some brands now offer predictive diagnostics via IoT sensors to preempt failures and extend warranty effectiveness. |
| 5. How are firmware updates and software integration handled under warranty for smart dental chairs? | Premium dental chairs in 2026 feature embedded IoT platforms that support over-the-air (OTA) firmware updates for ergonomic presets, safety protocols, and compatibility with new dental devices. These updates are included under warranty and managed through manufacturer cloud portals. Clinics must maintain an active service agreement to receive updates and technical support. Integration with practice management software (e.g., Open Dental, CareStack) is standardized via HL7/FHIR protocols, ensuring seamless data exchange. Unauthorized third-party modifications void software-related warranty coverage. |
Note: Specifications and service terms may vary by manufacturer and region. Always consult the official product documentation and service agreements before procurement.
Need a Quote for Most Expensive Dental Chair?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


