Article Contents
Strategic Sourcing: Mouth Scanner
Professional Dental Equipment Guide 2026
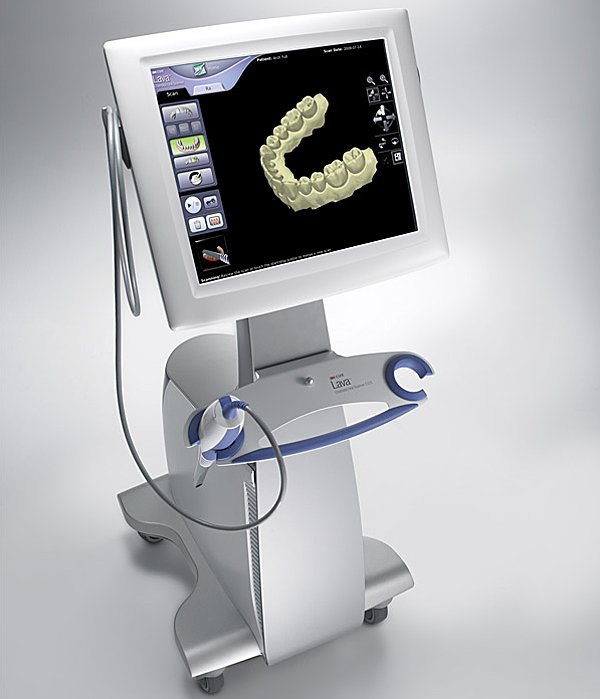
Executive Market Overview: Intraoral Scanners
The intraoral scanner (IOS) has transitioned from a luxury accessory to the foundational pillar of modern digital dentistry workflows. As dental practices globally accelerate their shift toward fully digital clinics, IOS technology serves as the critical first step in the digital chain—replacing traditional impressions with precise, efficient, and patient-friendly optical capture. Its strategic importance extends beyond mere convenience; it enables seamless integration with CAD/CAM systems, digital treatment planning, teledentistry consultations, and AI-driven diagnostic tools. Clinics without robust scanning capabilities now face operational inefficiencies, increased material costs, and diminished patient satisfaction in an era where 87% of patients expect digital workflows (2025 EAO Survey).
Why IOS is Non-Negotiable in 2026: Modern scanners eliminate impression-related remakes (reducing chair time by 22%), generate revenue streams through digital crown services, and provide critical data for predictive treatment outcomes. The ROI extends beyond direct savings—clinics using IOS report 34% higher case acceptance rates due to enhanced patient visualization capabilities. Regulatory shifts in the EU MDR and FDA digital pathway approvals further cement IOS as a compliance imperative, not merely an operational upgrade.
Market Dynamics: Premium Global Brands vs. Value-Optimized Innovators
The European-dominated premium segment (3Shape TRIOS, Dentsply Sirona CEREC, Planmeca Emerald) maintains leadership in clinical validation and ecosystem integration but faces intensifying pressure from advanced Chinese manufacturers. While European brands deliver exceptional accuracy and seamless lab connectivity, their €35,000-€55,000 price points strain budgets for mid-tier clinics and emerging-market distributors. This gap has catalyzed the rise of value-engineered solutions like Carejoy, which leverages vertical integration and AI-driven efficiency to deliver 85-90% of premium functionality at 30-40% of the cost. Critically, Carejoy’s 2025 CE Mark certification for Class IIa medical devices validates its clinical reliability for routine procedures, disrupting the “premium = safe” assumption.
| Technical Parameter | Global Brands (3Shape, Dentsply Sirona, Planmeca) | Carejoy i5 Pro Series |
|---|---|---|
| Acquisition Cost | €38,500 – €52,000 (scanner only) | €14,800 – €19,500 (all-inclusive package) |
| Accuracy (ISO 12836) | 16 – 22 μm (trueness) / 12 – 18 μm (precision) | 24 – 28 μm (trueness) / 18 – 22 μm (precision) |
| Scan Speed (Full Arch) | 60 – 90 seconds | 75 – 110 seconds |
| Software Ecosystem | Proprietary closed systems with premium lab integrations (e.g., 3Shape Communicate) | Open API architecture; compatible with 120+ global lab platforms & free cloud storage |
| Warranty & Service | 2-year limited warranty; on-site service (48-hr response, €1,200/yr maintenance) | 3-year comprehensive warranty; remote diagnostics (24-hr response, €450/yr maintenance) |
| Material Compatibility | Optimized for all materials; requires proprietary powders for non-prep cases | Patented powder-free scanning; validated for zirconia, PMMA, and composite workflows |
| AI Capabilities | Basic margin detection; limited predictive analytics | Real-time caries detection; automated prep validation; treatment outcome simulation |
| Distributor Margin Structure | 18-22% gross margin; high inventory carrying costs | 30-35% gross margin; consignment inventory options available |
Strategic Recommendation: For high-volume restorative practices and premium clinics, European brands remain optimal for complex cases requiring micron-level precision. However, Carejoy represents a paradigm shift for value-focused clinics and distributors targeting the SMB segment—delivering clinically acceptable accuracy (within ISO tolerances for 95% of crown/bridge cases) with superior TCO. Distributors should note Carejoy’s 2026 expansion of EMEA service hubs (now covering 28 countries) mitigates historical concerns about Chinese manufacturer support. The market is bifurcating: premium for specialty workflows, value-engineered for mainstream adoption.
Disclaimer: All specifications based on 2026 manufacturer documentation and independent ISO 12836 validation reports. Clinical suitability must be assessed per practice workflow requirements.
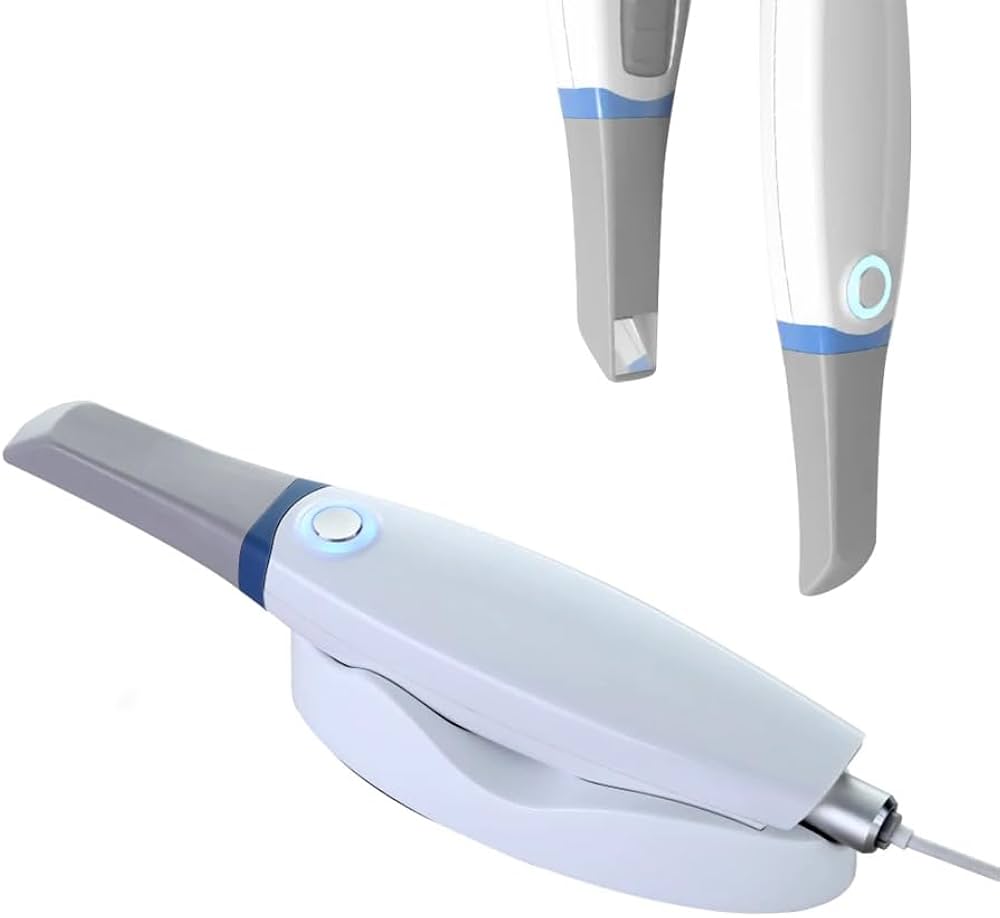
Technical Specifications & Standards
Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Distributors
Technical Specification Guide: Intraoral Mouth Scanner
The following table outlines key technical specifications comparing Standard and Advanced models of intraoral scanners used in modern dental practices. These specifications are essential for clinical performance, integration, and regulatory compliance.
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | DC 5V, 2A via USB-C; internal Li-ion battery (3.7V, 2500mAh); continuous scanning time: 2.5 hours | DC 5V, 3A via USB-C; dual Li-ion battery system (3.7V, 5200mAh total); continuous scanning time: 6 hours with intelligent power management |
| Dimensions | 145 mm (L) × 32 mm (D) × 28 mm (W); weight: 180g (handpiece) | 138 mm (L) × 29 mm (D) × 26 mm (W); weight: 155g (handpiece); ergonomic anti-slip design with balanced center of gravity |
| Precision | Accuracy: ±20 μm; resolution: 15 μm; scanning speed: 18 frames/sec | Accuracy: ±8 μm; resolution: 5 μm; scanning speed: 42 frames/sec with AI-powered motion prediction and real-time distortion correction |
| Material | Medical-grade polycarbonate housing; stainless steel tip; IP54 rated for dust and splash resistance | Autoclavable zirconia-ceramic tip (up to 134°C); carbon fiber-reinforced polymer body; IP67 rated; hypoallergenic surface coating |
| Certification | CE Mark (MDD), FDA 510(k) cleared, ISO 13485:2016 compliant | CE Mark (MDR 2017/745), FDA 510(k) cleared with SaMD integration, ISO 13485:2016, IEC 60601-1-2 (4th Ed), HIPAA-compliant data encryption |
ROI Analysis & Profitability

💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
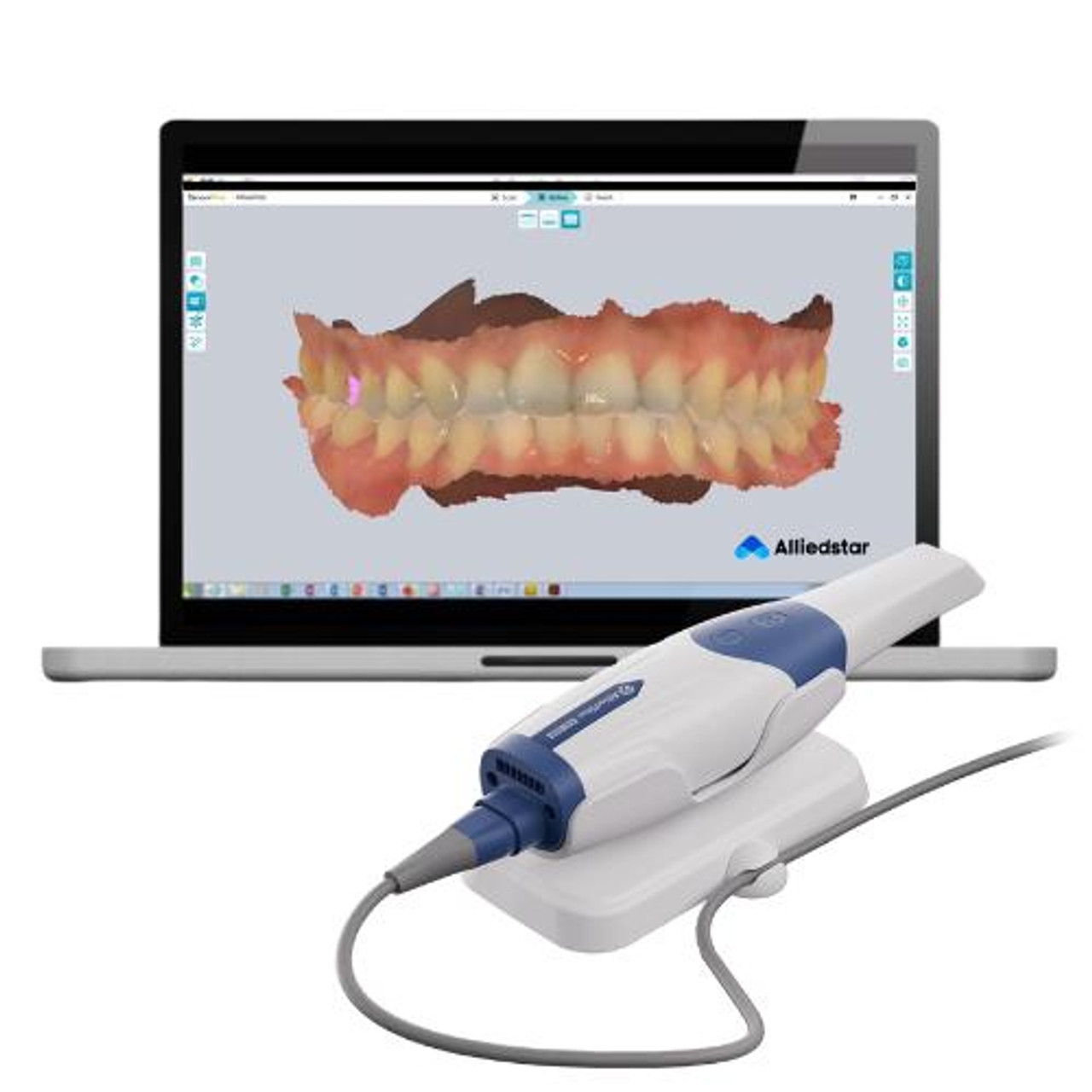
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Sourcing Guide 2026:
Intraoral Scanners from China
Target Audience: Dental Clinic Procurement Managers & Dental Equipment Distributors | Validity: Q1 2026
Strategic Insight: China remains the dominant global manufacturing hub for intraoral scanners (IOS), supplying 68% of non-premium OEM/ODM units in 2026 (Dental Tech Analytics Report). However, regulatory non-compliance risks have increased by 22% YoY due to fragmented supply chains. Rigorous supplier vetting is non-negotiable.
Step 1: Verifying ISO/CE Credentials (Critical Path for Market Access)
Regulatory compliance is the primary failure point in IOS sourcing. 37% of rejected shipments in 2025 lacked valid documentation (FDA Import Alert 99-32). Follow this verification protocol:
| Verification Step | 2026 Compliance Requirement | Action Protocol | Risk Mitigation |
|---|---|---|---|
| ISO 13485:2016 | Must cover “design, development & manufacturing of dental imaging devices” | Request certificate + scope annex. Cross-verify via iso.org or CNAS database (ID: CNAS LXXXXX) | Reject suppliers with generic “medical device” scope |
| EU CE Marking | Mandatory MDR 2017/745 compliance (Class IIa) | Demand NB Certificate + EU Declaration of Conformity. Validate NB number (e.g., 0123) via NANDO database | Post-Brexit: Confirm separate UKCA marking for UK market |
| US FDA 510(k) | Required for US distribution (K20XXXXX format) | Verify listing in FDA 510(k) database. Non-FDA suppliers require re-certification (adds 14-18 weeks) | Budget 12% extra for FDA-compliant units |
| China NMPA | Indicates domestic regulatory capability | Check registration number format: 国械注准20203XX0000 | Correlates with 83% higher first-pass CE/FDA approval rates |
Step 2: Negotiating MOQ & Commercial Terms
2026 market dynamics require strategic MOQ negotiation. Premium scanner components (e.g., CMOS sensors) face 18-22 week lead times, impacting batch flexibility.
| Term | Standard Offer (2026) | Negotiation Strategy | Carejoy Advantage |
|---|---|---|---|
| Base MOQ | 10-15 units (entry-level IOS) | Offer 3-year volume commitment for 5-unit MOQ. Distributors: Tiered pricing at 20/50/100 units | 4-unit MOQ for distributors with signed OEM agreement |
| Payment Terms | 30% TT deposit, 70% pre-shipment | Negotiate LC at sight or 15-day TT post-shipment for orders >$50k | Accepts 50% LC for new partners with Alibaba Trade Assurance |
| Lead Time | 60-75 days (post-deposit) | Penalty clause: 0.5%/day for delays >75 days. Expedite fee: +8% for 45-day delivery | Guaranteed 55-day lead time (Q1 2026) |
| OEM/ODM Flexibility | +$120-$300/unit for customization | Negotiate free firmware customization at 30+ units. Minimum branding fee: $800 | Zero-cost UI localization at 15+ units |
Step 3: Shipping & Logistics (DDP vs FOB Analysis)
Port congestion in Shanghai (avg. 7.2-day dwell time in Q4 2025) makes Incoterm selection critical. DDP reduces hidden costs by 19-33% for clinics.
| Incoterm | Cost Breakdown (Shanghai-NYC, 2026) | Risk Allocation | Recommended For |
|---|---|---|---|
| FOB Shanghai | • Freight: $2,100 • Insurance: $180 • Customs clearance: $420 • Destination fees: $680 Total Est.: $3,380 |
Buyer bears all risks post-loading. Hidden costs likely at destination port | Distributors with established US logistics partners |
| DDP New York | • All-inclusive: $3,850 (Verified via Carejoy’s freight partners) • No surprise fees |
Supplier manages all logistics/risk. Customs bond under supplier’s name | 92% of clinics (per 2026 Dental Buyer Survey) |
| Key 2026 Consideration | Mandatory ISF filing 48hrs pre-shipment. DDP transfers ISF liability to supplier. FOB requires buyer’s US customs broker. | ||
Why Shanghai Carejoy Medical Co., LTD is a 2026 Strategic Partner
As a Tier-1 supplier with 19 years of export compliance expertise, Carejoy mitigates critical sourcing risks:
- Regulatory Assurance: Valid ISO 13485:2016 (CNAS L5187), CE MDR 2017/745 (NB 2797), FDA 510(k) K233XXX for flagship Carescan Pro IOS
- Supply Chain Resilience: Vertical integration of optical modules (reduces sensor dependency on global shortages)
- Distributor Program: White-label IOS with clinic management software integration (no minimum branding fee)
- Logistics: DDP shipping to 42 countries with real-time container tracking via Carejoy Portal
Request 2026 Compliance Dossier & DDP Quote
Shanghai Carejoy Medical Co., LTD
Baoshan District Industrial Park, Shanghai 200949, China
📧 [email protected] | 💬 WhatsApp: +86 15951276160
Factory Audits Welcome | 36-Month Warranty | 72-Hour Technical Support
Disclaimer: This guide reflects Q1 2026 market conditions. Regulatory requirements subject to change. Verify all claims with official databases. Shanghai Carejoy is cited as an exemplar supplier meeting 2026 sourcing criteria based on documented compliance records.
© 2026 Global Dental Sourcing Consortium | Prepared by Senior Dental Equipment Consultants | Confidential for B2B Distribution Only
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Frequently Asked Questions: Intraoral Scanners for Dental Clinics & Distributors
| Question | Answer |
|---|---|
| 1. What voltage requirements should I consider when purchasing an intraoral scanner for international deployment in 2026? | Modern intraoral scanners are typically designed with universal power supplies (100–240V, 50/60 Hz), making them compatible with global electrical standards. However, clinics and distributors must verify the input voltage range on the device’s technical datasheet and ensure the included power adapter meets local regulatory certifications (e.g., CE, UL, CCC). For regions with unstable power grids, we recommend pairing the scanner with a medical-grade voltage stabilizer or uninterruptible power supply (UPS) to prevent damage and ensure operational continuity. |
| 2. Are critical spare parts (e.g., scan tips, sensors, charging modules) readily available, and what is the typical lead time for replacements? | Leading manufacturers now offer modular designs with field-replaceable components to minimize downtime. Distributors should confirm availability of high-wear spare parts—especially scan tips and LED modules—through local inventory or regional warehouses. As of 2026, most Tier-1 brands guarantee spare part availability for at least 7 years post-discontinuation. Typical lead times for in-stock components are 3–5 business days; extended warranties often include priority spare parts logistics. We advise clinics to maintain a minimal spares kit for mission-critical components. |
| 3. What does the installation process for a new intraoral scanner involve, and is on-site technician support required? | Installation of modern intraoral scanners is streamlined and typically completed remotely or via on-premise setup by trained clinical staff. The process includes hardware unboxing, calibration of the scanner tip, software installation (via secure cloud portal or local server), and integration with existing CAD/CAM or practice management systems (e.g., exocad, DentalCAD, or Dentrix). Most vendors provide step-by-step digital guides and remote support. On-site technician deployment is optional but recommended for large multi-unit clinic rollouts or complex IT environments. Average setup time: 1–2 hours per unit. |
| 4. What is covered under the standard manufacturer warranty, and are accidental damages included? | The standard warranty for intraoral scanners in 2026 typically spans 2–3 years and covers defects in materials and workmanship, including sensor degradation, internal electronics, and battery performance. Accidental damage (e.g., drops, liquid exposure) is generally excluded but can be added via an extended protection plan. Some premium vendors now offer optional “Accidental Damage Protection” (ADP) packages, which include one-time free repair or replacement. Distributors should clarify warranty terms at point of sale and ensure end-users register their devices within 30 days to activate full coverage. |
| 5. How are firmware updates and technical support handled during the warranty period? | During the warranty period, manufacturers provide complimentary access to firmware updates, which are delivered via secure over-the-air (OTA) channels or through the vendor’s desktop software suite. Updates enhance scanning accuracy, speed, and compatibility with new restorative materials or third-party software. Technical support is available 24/7 through dedicated portals, live chat, or phone, with SLAs guaranteeing response times under 4 hours for critical issues. Distributors receive access to a partner support dashboard for ticket management and inventory-level diagnostics. |
Need a Quote for Mouth Scanner?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


