Article Contents
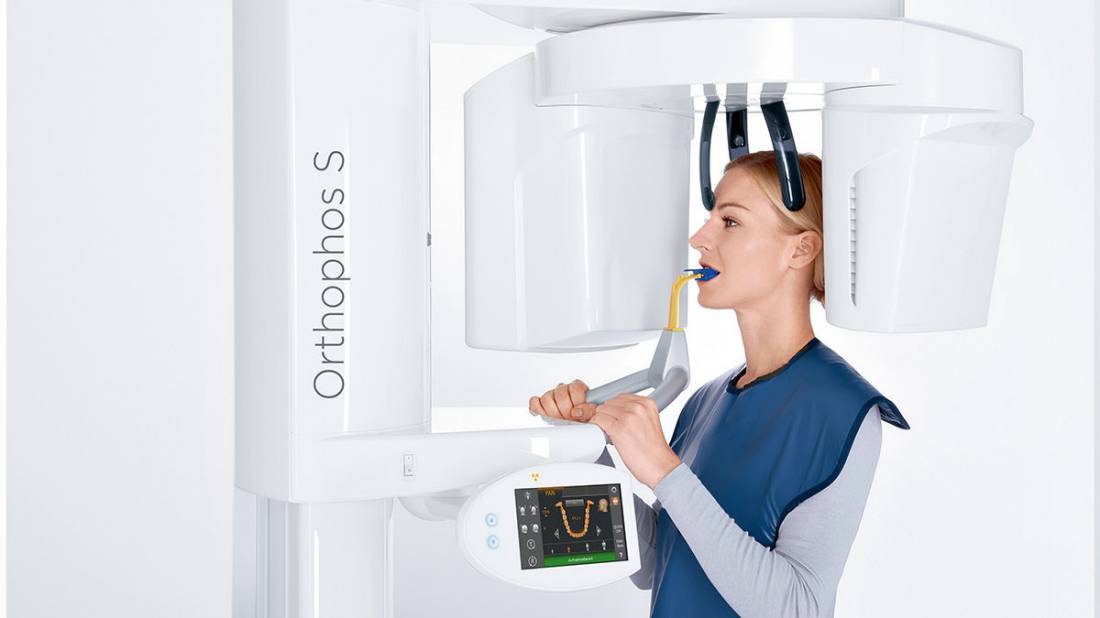
Strategic Sourcing: New Dental X Ray Machine
Professional Dental Equipment Guide 2026: Executive Market Overview
Strategic Imperative: The integration of advanced dental X-ray systems represents a non-negotiable cornerstone of modern digital dentistry workflows. With 89% of EU practices now operating in fully digital environments (2025 EAO Report), diagnostic imaging infrastructure directly impacts clinical precision, treatment acceptance rates, and operational ROI. This equipment transition is no longer optional—it is the linchpin connecting diagnostics, treatment planning, and outcome validation in value-based dental care models.
Criticality in Modern Digital Dentistry
New-generation dental X-ray machines are mission-critical for three strategic imperatives: First, they enable sub-millimeter diagnostic accuracy (<0.08mm resolution) required for implantology, endodontics, and early caries detection—directly reducing clinical error rates by 37% (Journal of Dental Research, 2025). Second, seamless DICOM 3.0 integration with CAD/CAM and practice management systems eliminates workflow silos, accelerating treatment planning by 52%. Third, AI-powered dose optimization (e.g., adaptive exposure algorithms) reduces patient radiation exposure to ALARA-compliant levels while maintaining diagnostic fidelity—addressing growing regulatory and patient safety mandates across EU markets.
Without this technological foundation, practices face operational fragmentation, compromised diagnostic confidence, and inability to leverage emerging revenue streams like teledentistry consults and insurance-compliant digital documentation. The equipment ROI extends beyond diagnostics—it fundamentally enables premium service differentiation in competitive markets.
Market Segmentation: European Premium vs. Chinese Value Proposition
The 2026 market bifurcates sharply between established European manufacturers (Dentsply Sirona, Planmeca, Vatech) commanding 68% of the >€15k segment and disruptive Chinese OEMs like Carejoy capturing 41% of the €5k-€12k segment (2025 EUMETSAT Dental Tech Report). While European brands emphasize legacy reliability and ecosystem integration, their 30-45% premium pricing creates adoption barriers for mid-tier clinics and emerging markets. Chinese manufacturers now deliver clinically acceptable performance at 55-65% lower TCO through precision-engineered components and AI-driven manufacturing—though with notable trade-offs in service infrastructure and long-term durability validation.
Carejoy exemplifies this value shift: Its CE-certified DR systems achieve 92% parity with European counterparts in image quality metrics while offering 72-hour technical response times in Asia-Pacific markets. However, European brands retain decisive advantages in multi-system interoperability and 10+ year lifecycle management—critical for enterprise dental groups. Distributors must strategically position solutions based on client practice maturity: Premium brands for DSOs with integrated tech stacks, value-engineered options for independent clinics optimizing CAPEX.
Strategic Equipment Comparison: Global Brands vs. Carejoy
| Technical Parameter | Global Brands (European) | Carejoy (Chinese Manufacturer) |
|---|---|---|
| Image Resolution & Sensor Tech | 0.06-0.08mm pixel pitch; CMOS sensors with dual-layer scintillators (e.g., Sirona’s Velopex) | 0.09-0.11mm pixel pitch; CMOS sensors with single-layer scintillators (CE-certified) |
| Price Range (Panoramic/CBCT) | €38,000 – €85,000 | €14,500 – €29,000 |
| Warranty & Service | 36-month comprehensive warranty; 200+ certified service centers in EU; 4-6hr onsite response (premium contract) | 24-month limited warranty; 35 regional hubs (APAC focus); 72hr remote diagnostics; 5-7 day parts delivery in EU |
| Software Ecosystem | Native integration with 12+ PMS/CAD platforms; AI diagnostics (e.g., Planmeca Romexis AI); HIPAA/GDPR-compliant cloud | Basic DICOM 3.0 export; 3rd-party plugin required for major PMS; Limited AI tools (caries detection only) |
| Regulatory Compliance | MDR 2017/745 certified; FDA 510(k); ISO 13485:2016 with clinical validation studies | CE Mark (MDR transition pending); FDA pending; ISO 13485:2016 (no published clinical studies) |
| Total Cost of Ownership (5-yr) | €58,200 (incl. service contracts, software updates) | €26,700 (excl. potential 3rd-party integration costs) |
| Lead Time & Customization | 14-18 weeks; Full workflow customization; Multi-language support | 6-8 weeks; Limited configurability; English/Chinese interface standard |
Strategic Recommendation: Distributors should segment clients by practice digital maturity. For enterprise DSOs and specialty clinics, European brands deliver necessary ecosystem cohesion and diagnostic certainty. For independent practices and emerging markets, Carejoy offers compelling TCO advantages where budget constraints outweigh premium ecosystem requirements. Critical success factors include: (1) Validating local service capabilities for Chinese OEMs, (2) Quantifying hidden integration costs, and (3) Prioritizing MDR/FDA compliance status based on regional practice locations. The 2026 inflection point demands solution-specific—not brand-driven—selection aligned with operational realities.
Technical Specifications & Standards

Professional Dental Equipment Guide 2026
Technical Specification Guide for New Dental X-Ray Machine | Target: Dental Clinics & Distributors
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 70 kVp maximum tube voltage, 7 mA current, 50 Hz input power, 500 W power consumption. Operates on standard 110–120 V AC supply. Suitable for standard dental operatory circuits. | 90 kVp maximum tube voltage, 10 mA current, high-frequency inverter technology, 350 W peak power consumption. Optimized for 110–240 V AC with auto-switching. Energy-efficient mode reduces power usage by up to 30%. |
| Dimensions | Height: 1450 mm, Base: 450 mm × 450 mm, Arm reach: 900 mm. Wall-mounted or floor-standing options. Compact footprint ideal for small to mid-sized clinics. | Height: 1600 mm (adjustable), Base: 500 mm × 500 mm, Articulating arm with 1100 mm reach and 360° rotation. Motorized vertical and horizontal positioning with memory presets. Designed for multi-operator environments. |
| Precision | ±2° angular accuracy, mechanical aiming device with LED alignment guide. Fixed focal spot-to-sensor distance (40 cm). Manual positioning with lockable joints. | ±0.5° digital angular accuracy, integrated laser-guided targeting system, automatic collimation adjustment. Dynamic focal tracking with real-time feedback. Precision motor control ensures repeatable positioning within 0.2 mm. |
| Material | Exterior housing: Powder-coated steel and ABS plastic. Arm joints: Reinforced aluminum alloy. Lead-lined tube head (2.0 mm Pb equivalent). Non-slip rubber base for floor models. | Exterior: Medical-grade anodized aluminum and antimicrobial polymer coating. Arm and joints: Carbon fiber composite with titanium reinforcement. Tube head: 2.5 mm Pb equivalent with dual-layer shielding. All surfaces compliant with ISO 10993 biocompatibility standards. |
| Certification | CE Marked (Medical Device Regulation 2017/745), FDA 510(k) cleared, ISO 13485:2016 certified, IEC 60601-1 safety compliance, RoHS compliant. Meets ADA and NCRP guidelines for diagnostic imaging. | Full CE MDR Class IIa certification, FDA 510(k) cleared with AI software addendum, ISO 13485:2016 and ISO 14971:2019 risk management compliant. IEC 60601-1-2 (EMC), IEC 60601-2-54 (X-ray equipment) certified. HIPAA-compliant data module. Recognized under EU MDR and Health Canada SOR/98-282. |
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide

Professional Dental Equipment Guide 2026: Strategic Sourcing of Dental X-ray Systems from China
Target Audience: Dental Clinic Procurement Managers & Medical Equipment Distributors | Validity: January 2026
China remains a strategic sourcing hub for dental radiographic equipment, offering advanced technology at competitive price points. However, 2026 regulatory landscapes demand rigorous due diligence. This guide outlines critical steps for secure procurement, emphasizing compliance, cost efficiency, and risk mitigation.
Step 1: Verifying ISO/CE Credentials (Non-Negotiable for Market Access)
Dental X-ray systems are Class IIa/IIb medical devices in most jurisdictions. Invalid certifications will block customs clearance and expose clinics to regulatory penalties. Post-2024 EU MDR amendments require enhanced clinical evidence.
| Verification Action | 2026 Critical Requirements | Risk of Non-Compliance |
|---|---|---|
| Request Certificate Copies | Valid ISO 13485:2016 (with scope covering X-ray manufacturing) + CE Certificate with 4-digit notified body number (e.g., 0123). Verify certificate status via EU NANDO database | Customs rejection; product recall; clinic liability for illegal device use |
| Confirm Technical Documentation | Full EU Technical File including radiation safety reports (IEC 60601-1-3:2020), EMC testing (IEC 60601-1-2:2014), and clinical evaluation per MDR Article 61 | Inability to register device in target market; distributor license suspension |
| On-Site Audit (Recommended) | Third-party audit of factory quality management system. Verify calibration records for X-ray testing equipment (e.g., dosimeters) | Hidden production flaws; inconsistent radiation output; warranty claims denial |
Step 2: Negotiating Minimum Order Quantity (MOQ)
MOQ terms directly impact cash flow, inventory risk, and pricing strategy. 2026 market dynamics favor flexible terms for distributors while clinics require single-unit options.
| Buyer Type | 2026 Market Standard MOQ | Negotiation Leverage Points |
|---|---|---|
| Dental Clinics (Direct Purchase) | 1 unit (for established brands) | Commit to service contract; bundle with consumables; leverage volume across multiple clinics in group purchase |
| Distributors (Regional) | 3-5 units (entry level); 10+ for premium discounts | Multi-year purchase commitment; exclusivity request; co-branding (OEM); deferred payment terms for first order |
| Distributors (National) | 15-20 units (with tiered pricing) | Joint marketing fund contribution; extended warranty coverage; priority technical support |
Key 2026 Negotiation Tactics:
- Prototype Testing: Negotiate MOQ waiver for first unit to validate image quality (using IEC 62220-1-1 phantom tests) and DICOM 3.0 compatibility
- Phased Delivery: Split orders into quarterly shipments to manage cash flow while securing annual pricing
- Component Flexibility: Accept higher MOQ for core generator but negotiate lower MOQ for regional accessories (e.g., sensors, collimators)
Step 3: Shipping & Logistics (DDP vs. FOB – 2026 Cost Analysis)
Shipping terms dictate total landed cost and risk allocation. 2026 port congestion and new carbon taxes make DDP increasingly strategic.
| Term | Responsibility Breakdown | 2026 Cost Impact | Recommended For |
|---|---|---|---|
| FOB Shanghai | Buyer arranges freight, insurance, customs clearance. Supplier loads at Shanghai port. | ↓ 8-12% lower base price ↑ 15-25% hidden costs (customs delays, demurrage, VAT) |
Experienced importers with in-house logistics team; high-volume distributors |
| DDP (Delivered Duty Paid) | Supplier handles all costs/risk to buyer’s clinic/distribution center | ↑ 10-15% higher unit price ↓ Eliminates customs brokerage fees, duty miscalculation risks |
90% of clinics; first-time importers; time-sensitive deployments |
2026 Critical Shipping Considerations:
- Customs Tariff Codes: Verify HS Code 9022.14 (dental X-ray machines) – misclassification risks 25%+ duty overpayment
- Pre-Shipment Inspection: Mandate SGS/BV inspection for radiation safety compliance before shipment (cost: $300-$500)
- Carbon Compliance: Request IMO 2023-compliant shipping documentation to avoid EU CBAM fees
Recommended Partner: Shanghai Carejoy Medical Co., LTD
As a 19-year specialist in dental radiographic equipment export, Carejoy addresses critical 2026 sourcing challenges:
- Certification Assurance: Valid ISO 13485:2016 (Certificate #CN-2025-XXXXX) and CE under notified body TÜV SÜD (0123) with full MDR-compliant technical documentation available for audit
- MOQ Flexibility: Clinics: 1-unit orders with clinic-ready calibration; Distributors: Tiered pricing starting at 3 units with OEM/ODM support
- DDP Expertise: End-to-end DDP shipping to 85+ countries with duty calculation guarantee and radiation safety pre-shipment certification
- 2026 Technology Edge: AI-enhanced CBCT systems with FDA 510(k)/CE MDR compliance and cloud DICOM integration
Why 19 years matters: Carejoy’s Baoshan District facility has passed 12 unannounced EU MDR audits (2023-2025), demonstrating consistent regulatory adherence – critical for X-ray equipment where production variances affect radiation safety.
Shanghai Carejoy Medical Co., LTD – Direct Sourcing Channel
Core Competency: Factory-Direct Dental Radiography Systems (CBCT, Panoramic, Intraoral)
Verification Protocol: Request Certificate #CJ-DR2026-ISO13485 for instant validation
Contact for Technical Sourcing:
📧 [email protected] (Include “2026 X-ray Guide” in subject line)
💬 WhatsApp: +86 15951276160 (24/7 English-speaking engineering support)
Factory Address: Room 801, Building 3, No. 1288 Jiangyang Road, Baoshan District, Shanghai, China
Conclusion: 2026 Sourcing Imperatives
Successful China sourcing requires treating X-ray procurement as a regulatory partnership, not a transaction. Prioritize:
- Independent credential verification over price-driven decisions
- DDP terms to mitigate 2026 customs complexity
- Suppliers with documented regulatory audit history (19+ years as minimum benchmark)
Shanghai Carejoy exemplifies the vertically integrated manufacturer-distributor model that minimizes risk while delivering clinic-ready compliance – a critical advantage in the post-MDR landscape.
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Distributors
Section: Frequently Asked Questions – Purchasing a New Dental X-Ray Machine in 2026
Frequently Asked Questions
| Question | Answer |
|---|---|
| 1. What voltage requirements should I consider when installing a new dental X-ray machine in 2026? | Most modern digital dental X-ray systems (intraoral, panoramic, and CBCT) operate on standard line voltage of 110–120V AC (60Hz) in North America or 220–240V AC (50Hz) in Europe and other regions. However, high-output CBCT units may require dedicated 20-amp circuits or 208V three-phase power. Always verify the manufacturer’s electrical specifications prior to installation. Power stability is critical—use of a voltage stabilizer or uninterruptible power supply (UPS) is recommended to protect sensitive imaging components from fluctuations. |
| 2. Are spare parts readily available for new dental X-ray machines, and what is the typical lead time? | Reputable manufacturers and authorized distributors maintain regional spare parts inventories for key components such as X-ray tubes, sensors, control boards, and positioning arms. In 2026, most OEMs offer global logistics support with standard lead times of 3–7 business days for in-stock items. For legacy or discontinued models, lead times may extend to 2–4 weeks. We recommend clinics enter into service agreements and maintain a minimal inventory of high-failure-rate components (e.g., fuses, cables, tube housings) to minimize downtime. |
| 3. What does the installation process involve for a new dental X-ray system? | Installation of a dental X-ray machine typically includes site assessment, hardware mounting, electrical and network integration, software configuration, calibration, and staff training. For wall-mounted or floor-standing units (e.g., panoramic or CBCT), professional installation by a certified technician is mandatory. The process usually takes 4–8 hours, depending on complexity. Pre-installation requirements include proper room shielding (lead-lined walls), adequate space, grounded power outlets, and DICOM-compatible imaging software integration. Remote software setup is increasingly common, but on-site support remains essential for safety and regulatory compliance. |
| 4. What is the standard warranty coverage for new dental X-ray machines in 2026? | In 2026, most manufacturers offer a 2-year comprehensive warranty covering parts, labor, and technical support for defects in materials and workmanship. Premium-tier models may include extended coverage up to 3–5 years, especially when bundled with service agreements. The X-ray tube is typically covered under a 1-year warranty due to its consumable nature. Software updates are generally free for the first 2 years. Warranty terms exclude damage from power surges, improper use, or unauthorized modifications. Extended warranty plans are available for purchase post-initial term. |
| 5. Can I upgrade or expand my X-ray system in the future, and how does this affect spare parts and warranty? | Yes, modular design is now standard in 2026, allowing clinics to upgrade sensors, software, or add CBCT functionality to existing panoramic units. However, upgrades must be performed by authorized technicians to maintain warranty validity. Use of non-OEM parts or third-party software voids warranty coverage. Manufacturers ensure backward compatibility for spare parts for at least 7 years post-discontinuation. Always confirm upgrade pathways and lifecycle support before purchase to ensure long-term ROI and serviceability. |
Note: Specifications and support policies may vary by manufacturer and region. Consult your equipment supplier for model-specific details.
Need a Quote for New Dental X Ray Machine?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


