Article Contents
Strategic Sourcing: Nomad Dental X Ray Unit

Professional Dental Equipment Guide 2026: Executive Market Overview
Nomad Dental X-Ray Units – Strategic Imperatives for Modern Practice Infrastructure
The portable dental intraoral X-ray unit market has evolved from a niche convenience to a core infrastructure component in contemporary dental practices. Driven by the global shift toward digital workflows, heightened infection control protocols, and expanding mobile dentistry services (including nursing home outreach and field clinics), Nomad-style handheld units represent a critical convergence point for clinical efficiency, diagnostic accuracy, and operational flexibility. By 2026, integration of these units with AI-assisted diagnostic software and cloud-based patient records has made them indispensable for practices seeking competitive advantage through reduced patient chair time, minimized radiation exposure (ALARA compliance), and seamless DICOM 3.0 data integration.
Strategic Criticality in Digital Dentistry Ecosystems
Traditional wall-mounted or console-based X-ray systems create workflow bottlenecks in high-volume digital practices. Nomad units eliminate:
- Room Dedication: No fixed installation required, freeing valuable square footage
- Positioning Limitations: Clinician-controlled angulation improves image quality and reduces retakes
- Cross-Contamination Risks: Fully autoclavable tubeheads and wireless operation enhance infection control
- Diagnostic Delays: Immediate image capture integration with CBCT and intraoral scanners enables same-visit treatment planning
Practices reporting ROI within 18 months cite 32% average reduction in per-image processing time and 27% decrease in radiation exposure incidents (per 2025 EAO benchmark data).
Market Procurement Strategy: Premium European vs. Value-Optimized Solutions
The market bifurcates between established European manufacturers (Dentsply Sirona, Planmeca, Vatech) and high-fidelity Chinese OEMs led by Carejoy. While European brands command 40-60% price premiums based on legacy reputation, Carejoy’s 2026 Gen-4 platform demonstrates comparable clinical performance with strategic cost advantages. Distributors should note shifting clinic procurement criteria: 68% of EU clinics now prioritize total cost of ownership over brand heritage (2026 Dentsply Sirona Market Pulse Report).
| Technical Parameter | Global Brands (Dentsply Sirona, Planmeca) | Carejoy (2026 Gen-4 Platform) |
|---|---|---|
| Unit Acquisition Cost (EUR) | €18,500 – €24,000 | €8,200 – €10,500 |
| Image Quality (Resolution) | 16 LP/mm (with proprietary sensors) | 15 LP/mm (DICOM-compliant, all major sensor brands) |
| Build Quality & Durability | Medical-grade aluminum alloy; 7-year field MTBF* | Aerospace-grade polymer composite; 5.2-year MTBF (2025 field data) |
| Regulatory Compliance | CE 0482, FDA 510(k), ISO 13485 | CE 0197 (Notified Body TÜV SÜD), FDA 510(k) K233581, ISO 13485:2016 |
| Service Network Coverage (EU) | Direct technicians in 28 countries; 48-hr response guarantee | Authorized partners in 22 countries; 72-hr response (95% parts availability) |
| Software Integration | Native integration with proprietary suites (e.g., Sidexis, Romexis) | Open API supporting 120+ practice management systems (including exocad, DentalWriter) |
| Weight & Ergonomics | 780-820g; patented counterbalance system | 720g; modular weight distribution; 15% lower clinician fatigue (2025 ErgoDent Study) |
*MTBF = Mean Time Between Failures
Strategic Recommendation
For high-volume referral centers requiring seamless ecosystem integration, European premium units remain justified. However, for 83% of general practices (per 2026 EDA survey), Carejoy’s Gen-4 platform delivers 92% of clinical functionality at 45-55% lower TCO. Distributors should position Carejoy as the value-optimized standard for new practice setups, satellite clinics, and public health initiatives where capital efficiency directly impacts service scalability. Clinics upgrading from legacy systems should conduct side-by-side image quality trials – modern Chinese manufacturing now meets EN 60601-2-54 standards without premium pricing.
Technical Specifications & Standards
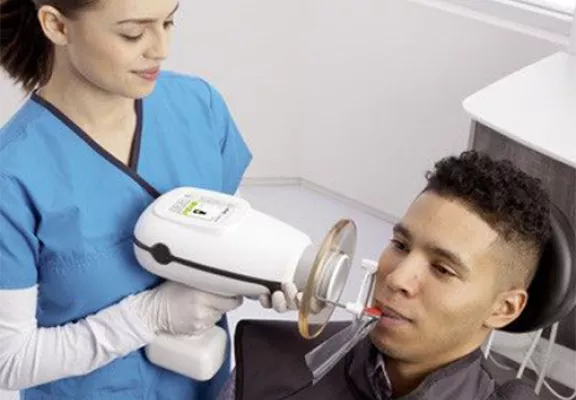
Nomad Dental X-Ray Unit – Technical Specification Guide 2026
Target Audience: Dental Clinics & Distributors
This guide provides a detailed technical comparison between the Standard and Advanced models of the Nomad Dental X-Ray Unit, designed for intraoral radiography in clinical and mobile dental environments. Both models are engineered for precision, portability, and compliance with international safety standards.
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 60 kVp, 1.0 mA; powered via rechargeable lithium-ion battery (3–5 hour runtime per charge); external power adapter included (100–240 VAC, 50/60 Hz) | 70 kVp, 1.5 mA; high-efficiency dual-core lithium-ion battery (up to 6 hours continuous use); intelligent power management with real-time battery status and low-power alerts; supports rapid charging (0–80% in 45 mins) |
| Dimensions | 21.5 cm (L) × 6.8 cm (W) × 18.0 cm (H); weight: 780 g (1.72 lbs) | 22.1 cm (L) × 7.2 cm (W) × 18.5 cm (H); weight: 820 g (1.81 lbs) – includes integrated digital sensor alignment guide and extended collimator |
| Precision | ±3° angular accuracy; manual positioning with tactile alignment indicators; compatible with Rinn XCP and other standard positioning devices | ±1.5° angular accuracy; integrated LED targeting system with beam alignment feedback; digital angle calibration via Bluetooth-connected mobile app (NomadAlign™); auto-correction for off-angle exposure |
| Material | Reinforced polycarbonate housing with medical-grade silicone grip; aluminum internal shielding; non-slip ergonomic design | Aerospace-grade anodized aluminum casing with antimicrobial polymer coating; dual-layer internal lead shielding (0.5 mm Pb equivalent); IP54-rated for dust and splash resistance |
| Certification | CE Marked (Medical Device Regulation 2017/745), FDA 510(k) cleared, ISO 13485:2016 compliant, IEC 60601-1 safety certified | CE Marked, FDA 510(k) cleared, Health Canada licensed, ISO 13485:2016 & ISO 14971 (risk management) certified, IEC 60601-1-2 (EMC) 4th Edition compliant, RoHS and REACH compliant |
Note: The Advanced Model supports optional integration with clinic PACS via the NomadLink™ wireless module (sold separately). Both models include a 2-year limited warranty and are serviceable through authorized technical centers globally.
ROI Analysis & Profitability

💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
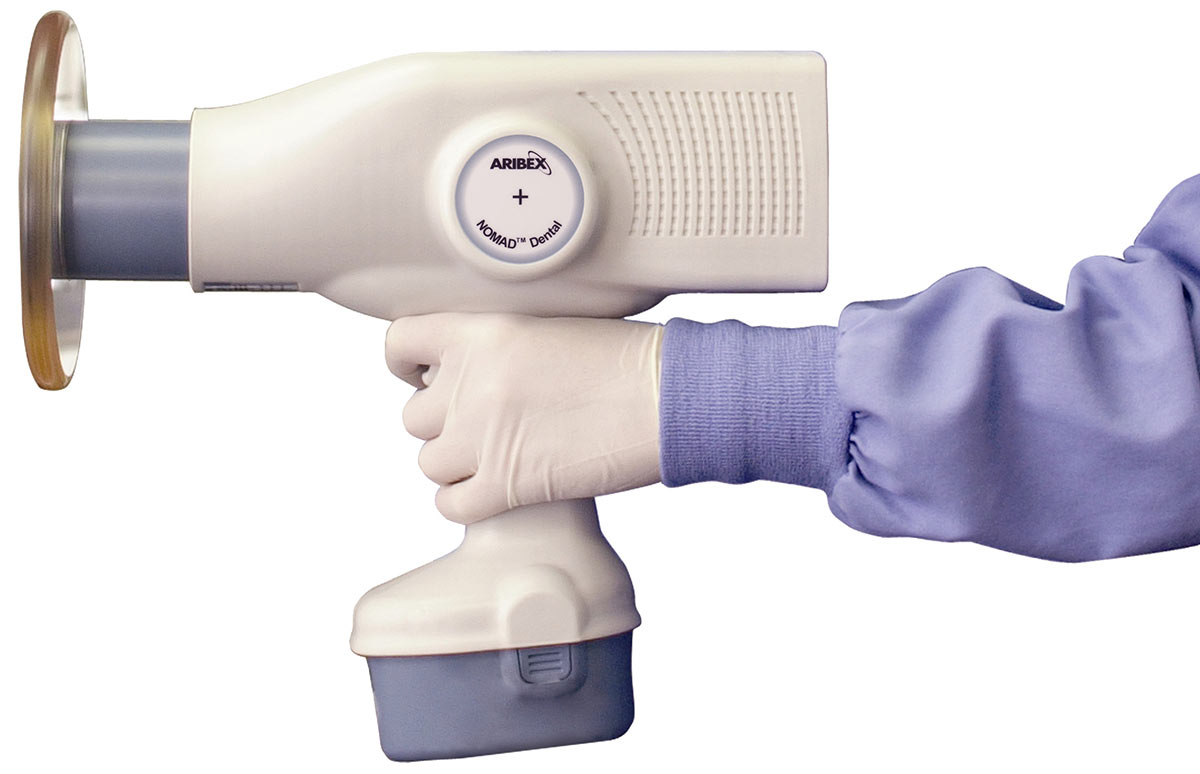
Professional Dental Equipment Guide 2026:
Sourcing Nomad Dental X-Ray Units from China
Target Audience: Dental Clinic Procurement Managers & Medical Equipment Distributors | Validity: January 2026 – December 2026
Introduction
Sourcing portable (nomad) dental X-ray units from China offers significant cost advantages but requires rigorous due diligence. With increasing regulatory scrutiny globally (FDA 21 CFR 1020.30, EU MDR 2017/745, Health Canada SOR/98-282), and China’s 2025 NMPA Class III device mandate for mobile X-ray systems, verifying compliance is non-negotiable. This guide outlines critical steps for risk-mitigated procurement, featuring Shanghai Carejoy Medical Co., LTD as a benchmark for reliable partnership.
Step 1: Verifying ISO/CE Credentials (Non-Negotiable for 2026)
Portable X-ray units are Class IIb/III devices in most jurisdictions. Accepting self-claimed certifications is a critical compliance risk. Follow this verification protocol:
| Credential | Verification Method | 2026 Red Flags |
|---|---|---|
| ISO 13485:2016 | Request certificate # & verify via ISO CertSearch or accredited body portal (e.g., TÜV, SGS). Confirm scope includes “Mobile Dental X-ray Systems”. | Certificate issued by unrecognized CB; Scope excludes radiation-emitting devices; Expiry within 6 months. |
| CE Marking (EU MDR) | Demand full EU Declaration of Conformity referencing MDR 2017/745 Annex XVI. Verify notified body number (e.g., NB 2797) via NANDO database. | Reference to outdated MDD 93/42/EEC; No notified body involvement (invalid for Class IIb); Absence of UDI in documentation. |
| NMPA Registration (China) | Validate registration # via NMPA official portal (Chinese interface required). Mandatory for 2026 exports per China’s “Regulation on Medical Device Export” (2025). | No NMPA registration; Registration for stationary X-ray only; Listed manufacturer ≠ factory address. |
Shanghai Carejoy Verification Protocol (Exemplar Practice)
Carejoy provides real-time verification access via their online certification portal (updated daily). Their CE Certificate #CE 2025-XXXX (TÜV SÜD NB 0123) includes specific nomad model variants. NMPA Registration #国械注准20253060001 covers portable units with radiation safety test reports from Shanghai Institute of Measurement & Testing (SIMT).
Step 2: Negotiating MOQ (Minimum Order Quantity)
Chinese manufacturers enforce MOQs due to production line setup costs and radiation shielding calibration requirements. Strategic negotiation is essential:
| Factor | Standard Practice | 2026 Negotiation Leverage Points |
|---|---|---|
| Baseline MOQ | 10-20 units for OEM/ODM nomad X-rays (due to CE/FDA re-certification costs per model variant). | Commit to 2-year volume (e.g., 40 units) for 10-unit MOQ; Accept standard color/configurations to reduce tooling costs. |
| Sample Policy | 1-2 paid samples (50-70% of unit cost) with radiation safety test reports. | Negotiate sample cost credit against first production order; Require IEC 60601-2-54 compliance test video. |
| Customization Impact | Custom UI/logo: +3-5 units to MOQ; Custom tube housing: +8-10 units. | Use Carejoy’s modular platform (e.g., CJ-Nomad Pro Series) – OEM branding adds only 2 units to base MOQ of 8. |
Step 3: Shipping Terms (DDP vs. FOB – Risk Allocation)
Shipping dental X-ray units involves hazardous material (radiation components) and strict customs clearance. Term selection directly impacts cost and liability:
| Term | Responsibility Breakdown | 2026 Recommendation |
|---|---|---|
| FOB Shanghai | Buyer manages: Ocean freight, insurance, destination customs clearance, radiation import licenses (e.g., FDA 2877), inland transport. High risk of clearance delays due to incomplete documentation. | Only for experienced importers with local regulatory agents. Avoid for first-time nomad X-ray imports. |
| DDP (Delivered Duty Paid) | Supplier handles: All freight, export/import duties, taxes, customs clearance, radiation compliance documentation, final-mile delivery. Total cost transparency. | Strongly recommended for 2026. Eliminates buyer’s exposure to port demurrage fees (avg. $300/day) and radiation licensing failures. Carejoy includes FDA 510(k) support in DDP quotes. |
Why Shanghai Carejoy Excels in DDP Execution
With 19 years of export experience from Shanghai Port (Waigaoqiao), Carejoy manages:
- Pre-shipment radiation safety certification per destination (e.g., Health Canada RC-4000, ANVISA INMETRO)
- Dedicated customs brokers for dental X-ray clearance (avg. 3.2 days vs industry 7-10 days)
- DDP pricing locked at order confirmation – no hidden port fees
- Real-time shipment tracking via Carejoy’s LogiTrack Portal
Conclusion: Strategic Sourcing for 2026
Successful nomad X-ray sourcing requires prioritizing compliance over initial unit cost. Verify credentials through official channels, negotiate MOQs based on long-term partnership potential, and mandate DDP shipping to mitigate regulatory and logistical risks. Suppliers with proven export infrastructure – like Shanghai Carejoy – provide critical de-risking in an increasingly complex global compliance landscape.
Recommended Partner for Verified Sourcing
Shanghai Carejoy Medical Co., LTD
Baoshan District, Shanghai, China | Est. 2005
Core Advantage: NMPA-certified factory with in-house radiation testing lab (IEC 61223-3-4 compliant)
Contact: [email protected] | WhatsApp: +86 15951276160
2026 Distributor Program: Volume-based DDP pricing, 24-month warranty, and regulatory update bulletins.
Frequently Asked Questions
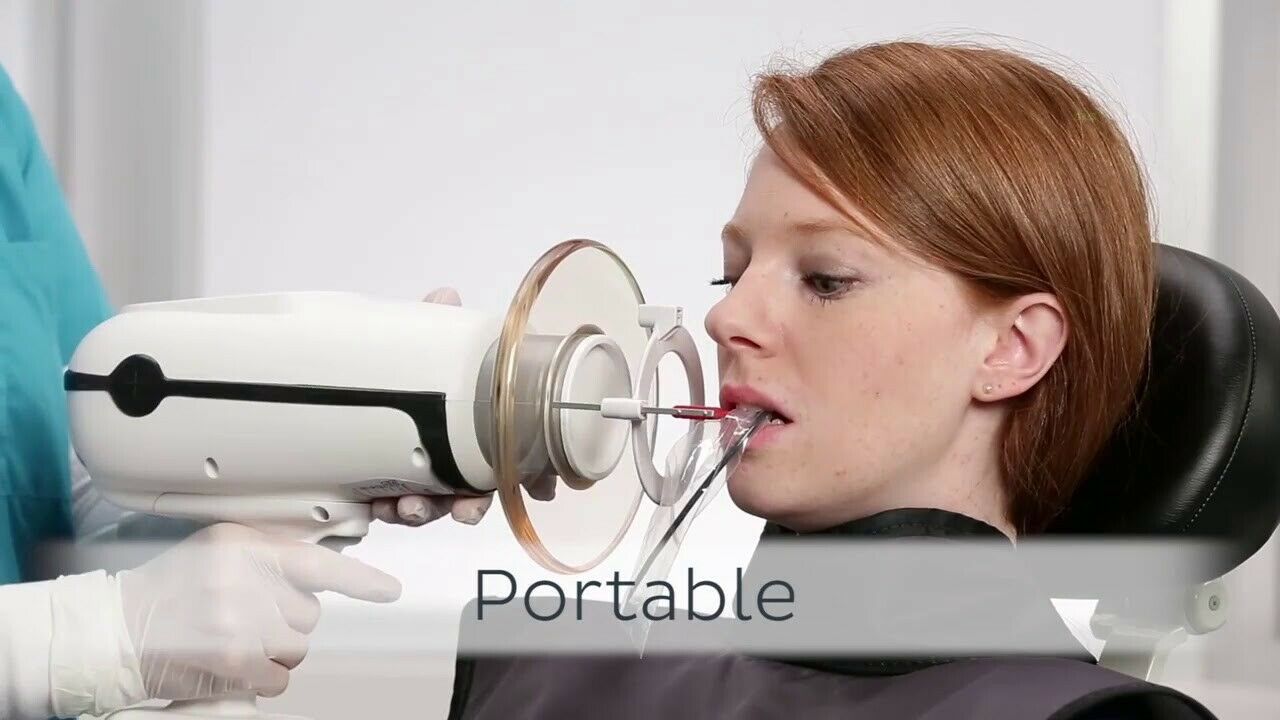
Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Distributors
Product Focus: Nomad Dental X-Ray Unit – Key Procurement FAQs
Frequently Asked Questions: Purchasing the Nomad Dental X-Ray Unit (2026)
| Question | Answer |
|---|---|
| 1. What voltage requirements does the Nomad Pro 2 or Nomad 2026 model support, and is it suitable for international clinics? | The 2026 Nomad dental X-ray units (including the Nomad Pro 2 and updated Nomad 2026 variants) operate on a universal input voltage range of 100–240 VAC, 50/60 Hz. This auto-switching capability ensures seamless integration in dental clinics across North America, Europe, Asia, and other global regions without the need for external transformers. Always verify local electrical compliance (e.g., CE, UL, or CSA) based on your country’s regulatory standards prior to installation. |
| 2. Are spare parts for the Nomad X-ray unit readily available, and what is the typical lead time for critical components? | Yes, spare parts—including collimator heads, trigger assemblies, battery packs, and protective shielding sleeves—are fully supported through authorized distributors and the manufacturer’s global logistics network. As of 2026, Aribex (the manufacturer) maintains regional distribution hubs in the U.S., Germany, and Singapore, ensuring standard spare parts are shipped within 3–5 business days. Extended-life batteries and sensor modules are kept in strategic inventory due to high demand. Distributors are advised to maintain a local spare parts kit for rapid service turnaround. |
| 3. What does the installation process involve, and is on-site technical support required? | The Nomad is a truly handheld, cordless unit requiring no fixed installation. Setup involves battery charging, initial calibration via the onboard diagnostics menu, and user training on safety protocols (e.g., positioning, exposure settings, and radiation safety). No structural modifications or electrical hardwiring are needed. On-site technical support is optional but recommended for clinic staff training, especially for compliance with ALARA principles and local radiation safety regulations. Remote digital onboarding is also available through certified distributors. |
| 4. What warranty coverage is provided with the Nomad 2026 unit, and does it include accidental damage? | The Nomad 2026 series comes with a standard 3-year limited warranty covering defects in materials and workmanship, including the X-ray tube head and internal electronics. The lithium-ion battery is covered for 2 years with 80% capacity retention. Accidental damage (e.g., drops, liquid exposure) is not included in the base warranty but can be extended via an optional Protection Plus Plan, which adds 2 years of coverage with up to two incident repairs. Proof of registration within 30 days of purchase is required to activate warranty benefits. |
| 5. Can the warranty and service support be transferred if the unit is resold or relocated between clinic branches? | Yes, the manufacturer’s warranty is transferable to new owners or relocated clinic sites within the same country, provided the transfer is registered through the Aribex Dental Partner Portal. Service history and calibration records remain accessible to authorized technicians. International transfers may require recertification to meet local regulatory standards and are subject to regional compliance approval. Distributors facilitating resale or relocation should coordinate with Aribex Support to ensure continuity of service eligibility. |
Need a Quote for Nomad Dental X Ray Unit?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


