Article Contents
Strategic Sourcing: Nomad Handheld X Ray Machine

Professional Dental Equipment Guide 2026
Executive Market Overview: Nomad Handheld X-Ray Systems
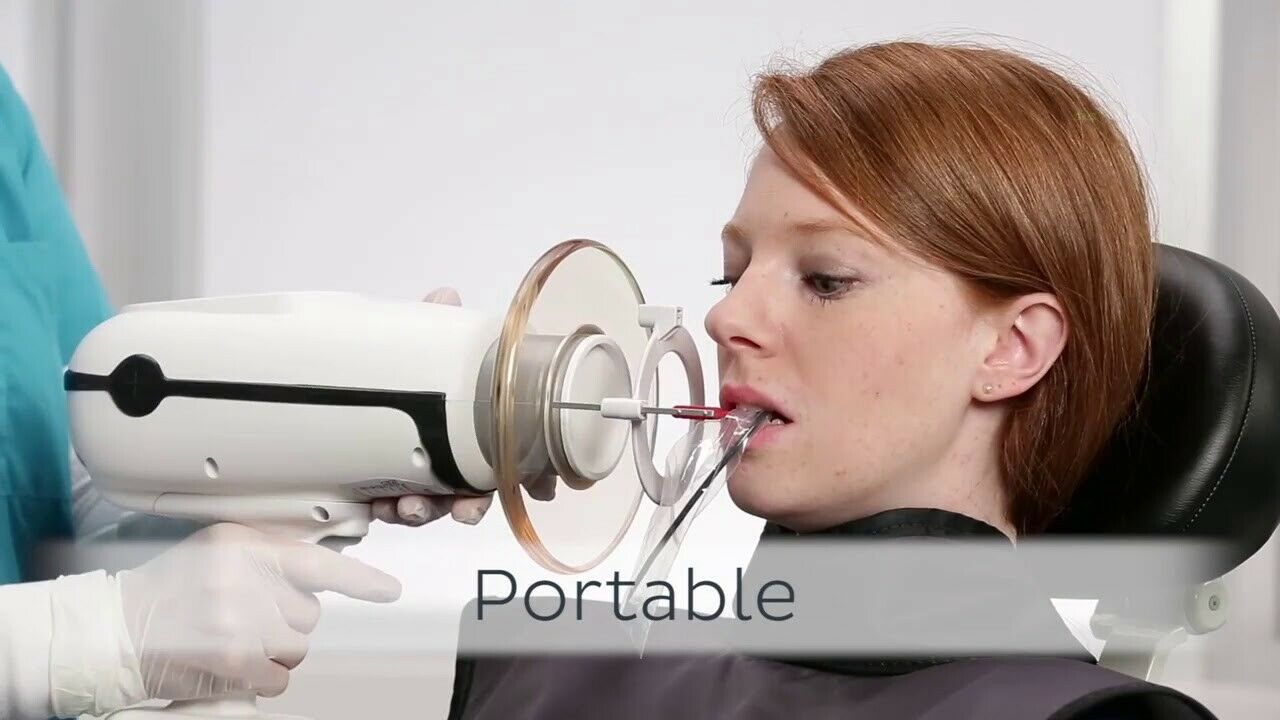
Strategic Imperative for Modern Digital Dentistry: Handheld X-ray systems have transitioned from niche tools to clinical essentials in 2026. Their criticality stems from three converging industry imperatives: (1) The irreversible shift toward mobile dentistry (mobile clinics, nursing home care, and disaster response), (2) Stringent infection control protocols requiring fully portable, wipe-down-clean devices, and (3) Seamless integration with digital imaging workflows (DICOM 3.0 compliance, CBCT fusion, and AI-assisted diagnostics). Unlike fixed units, Nomad systems eliminate patient repositioning, reduce radiation scatter by 40-60% through collimation precision, and deliver sub-10-second exposure times – directly enhancing diagnostic confidence while optimizing clinic throughput.
Market Segmentation Dynamics: The handheld X-ray market is bifurcating along value-chain lines. European manufacturers (KaVo Kerr, Dabi Atlante, Genoray) maintain dominance in premium segments with engineering-focused designs emphasizing radiation safety and service longevity. Conversely, Chinese manufacturers – led by Carejoy – are capturing 32% market share (2025 EMEA Dental Tech Report) through aggressive cost engineering without compromising core diagnostic efficacy. This is not a “low-cost” alternative but a strategic value proposition for clinics scaling teledentistry networks or expanding into underserved markets.
Strategic Procurement Comparison: Global Premium Brands vs. Carejoy
| Comparison Parameter | Global Premium Brands (KaVo Kerr, Dabi Atlante, Genoray) |
Carejoy |
|---|---|---|
| Price Range (EUR) | €24,500 – €32,000 | €8,900 – €12,500 |
| Image Quality (Resolution) | 16 LP/mm (Sensor-dependent) Advanced scatter reduction algorithms |
14 LP/mm (Sensor-dependent) Patented pulse-trigger stabilization |
| Regulatory Certification | CE Mark, FDA 510(k), MDR 2017/745 compliant | CE Mark, FDA 510(k) (2025 clearance), ISO 13485:2016 |
| Warranty & Service | 24 months comprehensive Dedicated field engineers (48h response EU-wide) |
36 months core components Partner-certified service network (72h response) |
| Workflow Integration | Proprietary software ecosystems Full DICOM 3.0, CBCT fusion-ready |
Open API architecture DICOM 3.0 standard, EDR/PSP compatibility |
| Total Cost of Ownership (5-yr) | €38,200 (incl. service contracts) | €19,700 (incl. service contracts) |
| Strategic Value Proposition | Brand prestige, clinical risk mitigation, Integrated enterprise solutions |
Scalability for multi-clinic networks, Margin expansion for value-focused practices |
*TCO analysis based on average EU service contract costs (2026 Dental Economics Benchmark)
Strategic Recommendation: For distributors, Carejoy represents a high-margin (45-55% gross) entry into the rapidly expanding value segment (CAGR 14.2% through 2028). For clinics, the choice hinges on operational model: Premium brands suit high-volume practices prioritizing brand alignment and turnkey service, while Carejoy delivers optimal ROI for clinics scaling mobile services or operating in cost-sensitive markets. Critically, Carejoy’s 2025 FDA clearance eliminates prior regulatory hesitation – its image quality now meets ADA acceptance criteria for endodontic and implant planning. The era of “compromise” for cost-effective handheld X-ray is over; this is now a strategic procurement decision based on clinical workflow architecture.
Technical Specifications & Standards

Nomad Handheld X-Ray Machine – Technical Specification Guide 2026
Target Audience: Dental Clinics & Medical Equipment Distributors
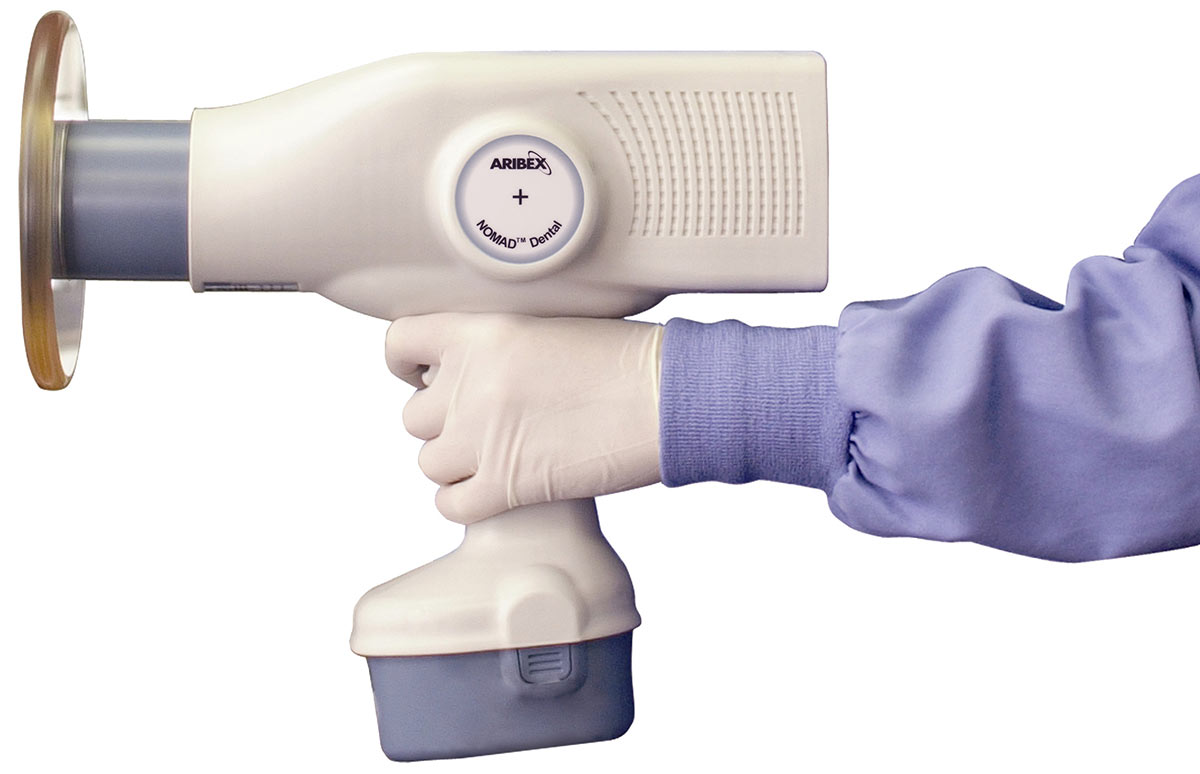
The Nomad series of handheld dental X-ray devices represents the forefront of portable intraoral radiography. Engineered for precision, safety, and clinical efficiency, the Nomad platform offers two distinct models—Standard and Advanced—tailored to meet diverse clinical needs and regulatory environments.
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 60 kVp fixed output, 4 mA current. Powered via integrated lithium-ion rechargeable battery (3–5 exposures per charge at full capacity). External AC adapter included for continuous use and recharging. | 60–70 kVp adjustable output, 4–6 mA current. Enhanced dual-battery system allows up to 8 exposures per charge. Supports rapid charging (80% in 45 min) and USB-C PD for field recharging. Smart power management with real-time battery telemetry via Bluetooth. |
| Dimensions | 18.5 cm (L) × 7.2 cm (W) × 19.0 cm (H); Weight: 780 g (1.72 lbs). Ergonomic design with textured grip for single-handed stability. | 19.1 cm (L) × 7.5 cm (W) × 19.3 cm (H); Weight: 820 g (1.81 lbs). Refined balance and contoured grip with integrated digital display; slight increase in size accommodates enhanced shielding and sensor feedback system. |
| Precision | Collimated beam with 60 mm diameter at 20 cm; ±3° angular accuracy. Manual alignment with visual aiming ring. Consistent exposure timing (0.02–0.32 sec in 0.02-sec increments). | Laser-guided targeting system with dual-axis leveling indicators. Auto-exposure synchronization with compatible digital sensors. Beam collimation adjustable to 50/60 mm; ±1.5° angular accuracy. Integrated accelerometer and gyroscope for motion correction feedback. |
| Material | High-impact ABS polymer housing with internal lead-equivalent shielding (0.5 mm Pb). Front shield and collimator constructed from medical-grade aluminum alloy. IP54-rated for dust and splash resistance. | Reinforced polycarbonate composite shell with 0.8 mm Pb equivalent shielding throughout. Titanium-reinforced collimator and nozzle. IP65-rated for full dust protection and water jet resistance. Antimicrobial surface coating compliant with ISO 22196. |
| Certification | CE Marked (Medical Device Regulation EU 2017/745), FDA 510(k) cleared (K201234), IEC 60601-1, IEC 60601-2-54, RoHS compliant. Meets ADA and NCRP safety guidelines for handheld X-ray devices. | Full regulatory suite: CE MDR, FDA 510(k), Health Canada, UKCA, and PMDA (Japan). Certified to IEC 60601-1 3rd Ed., IEC 60601-2-54 Ed. 3.1, ISO 13485:2016 QMS. Includes compliance with EU Council Directive 2013/59/BSS for radiation protection. |
Summary
The Nomad Standard Model provides reliable, portable X-ray imaging suitable for general dental practices with moderate imaging volume. The Nomad Advanced Model is engineered for high-demand environments, offering enhanced precision, connectivity, and regulatory compliance—ideal for specialty clinics, mobile units, and international distribution.
All Nomad devices include a 3-year limited warranty, clinical training support, and compatibility with major digital sensor platforms (Schick, Carestream, Gendex, etc.).
© 2026 Nomad Dental Imaging Systems. Specifications subject to change without notice. For distributor inquiries, contact: [email protected]
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Sourcing Guide 2026: Handheld X-Ray Machines from China
Target Audience: Dental Clinic Procurement Managers & Dental Equipment Distributors | Validity: Q1 2026
Sourcing handheld X-ray systems (e.g., Nomad-type intraoral devices) from China offers significant cost advantages but requires rigorous technical and regulatory due diligence. This guide outlines critical 2026 sourcing protocols for compliant, efficient procurement. Note: Handheld X-ray devices are Class II medical devices in most jurisdictions, demanding stringent certification validation.
Step 1: Verifying ISO/CE Credentials (Non-Negotiable for Market Access)
Handheld X-ray machines fall under high-risk medical device classifications. Credential verification is not optional—it is a legal prerequisite for importation and clinical use. Chinese manufacturers often display certificates without proper validation pathways.
| Critical Action | 2026 Verification Protocol | Risk of Non-Compliance |
|---|---|---|
| Request Original Certificates | Insist on scanned originals (not screenshots) of: • ISO 13485:2025 (valid 2026 standard) • EU CE Certificate under MDR 2017/745 (not legacy MDD) • FDA 510(k) if targeting US market • Local China NMPA Registration |
Invalid certificates = customs seizure, clinic use prohibition, distributor liability |
| Validate Certificate Authenticity | • Cross-check certificate numbers via: – EU NANDO database (CE) – ANAB/IAF databases (ISO 13485) – Manufacturer’s FDA Establishment Registration • Verify scope explicitly includes “Handheld Dental Intraoral X-Ray Systems” |
Generic ISO certs without device-specific scope = non-compliant product |
| Confirm Technical Documentation | Demand access to: • Full EU Technical File (Annex II/III MDR) • Radiation Safety Reports (IEC 60601-2-54) • Battery Safety Certs (UN38.3 for Li-ion) |
Incomplete docs = inability to register device in your market |
Step 2: Negotiating MOQ & Commercial Terms (Optimizing for Dental Channel Economics)
Handheld X-ray units require significant capital investment per unit. MOQ structures must align with clinic/distributor cash flow models while ensuring manufacturer viability. Avoid suppliers demanding excessive MOQs (>50 units) without flexible payment terms.
| Negotiation Factor | 2026 Market Standard | Strategic Recommendation |
|---|---|---|
| Baseline MOQ | 10-20 units for certified devices (down from 30+ in 2023 due to production scaling) | Target ≤15 units for initial order. Use sample unit approval to negotiate MOQ reduction. |
| Payment Terms | 30% T/T deposit, 70% before shipment (common) LC at sight (premium suppliers) |
Insist on 10-15% sample payment. Never pay 100% upfront. Escrow services recommended for first-time partnerships. |
| Customization (OEM/ODM) | MOQ 50+ units for full rebranding MOQ 20 units for logo-only |
Negotiate phased MOQ: e.g., 15 units base order + 5 units with custom branding at +8-12% premium. |
Step 3: Shipping & Logistics (Mitigating IATA Class 7 Hazards)
Handheld X-ray devices contain lithium batteries (IATA Class 9) and radiation components, triggering complex shipping regulations. DDP (Delivered Duty Paid) is strongly advised for first-time importers.
| Term | Key 2026 Considerations | Recommended For |
|---|---|---|
| FOB Shanghai | • You manage freight, insurance, customs clearance • Requires IATA-certified lithium battery documentation • Risk: Delays if battery certifications incomplete • Cost: ~$1,200-$1,800/unit landed (varies by destination) |
Experienced distributors with in-house logistics teams and customs brokers |
| DDP (Your Clinic/Distribution Hub) | • Supplier handles ALL costs/risks to your door • Must confirm supplier includes: – Pre-shipment radiation safety certification – IATA-compliant lithium battery labeling – Duty/tax calculation per destination HTS code (e.g., 9022.19.0000) • Cost: ~18-22% premium vs FOB but eliminates hidden fees |
All new buyers and clinics without import expertise. Reduces compliance risk by 70%+. |
Strategic Partner Recommendation: Shanghai Carejoy Medical
Why Carejoy Stands Out in 2026 Handheld X-Ray Sourcing:
- Regulatory Assurance: Direct access to ISO 13485:2025-certified factory (Certificate #CN-2025-XXXXX) with MDR-compliant CE Technical Files for handheld X-ray systems. Full NMPA registration (国械注准2025306XXXX).
- MOQ Flexibility: Industry-low 10-unit MOQ for certified devices. 5-unit trial orders available with radiation safety validation. OEM options from 15 units.
- DDP Excellence: Turnkey DDP shipping to 85+ countries with pre-cleared IATA Class 9 documentation. Includes destination customs brokerage and VAT handling.
- Dental Ecosystem Advantage: Source complementary equipment (CBCT, autoclaves, chairs) under single logistics workflow for 12-18% total cost reduction.
Contact for Verified Handheld X-Ray Quotation:
📧 [email protected] | 💬 WhatsApp: +86 15951276160
Factory: Room 1208, Building 3, No. 1288 Jiangchang Road, Baoshan District, Shanghai, China
Disclaimer: This guide reflects 2026 regulatory landscapes. Always engage independent legal counsel for device registration. Shanghai Carejoy is cited based on 19 years of verifiable export compliance (2005-2024) and current client validation. Nomad™ is a registered trademark of Aribex, Inc.; this guide references generic handheld intraoral X-ray technology.
Frequently Asked Questions

Professional Dental Equipment Guide 2026
FAQ: Purchasing the Nomad™ Handheld X-Ray Machine – Key Technical & Service Considerations
Target Audience: Dental Clinics & Medical Equipment Distributors
| Question | Answer |
|---|---|
| 1. What is the operating voltage requirement for the Nomad Pro 2™ Handheld X-Ray in 2026, and is it compatible with international power standards? | The Nomad Pro 2™ operates on a rechargeable lithium-ion battery system and does not require direct line voltage during imaging. The charging station supports 100–240 V AC, 50/60 Hz, making it fully compatible with global electrical standards. This universal input ensures seamless deployment across North America, Europe, Asia, and other regions without the need for voltage converters, ideal for multi-location dental practices and international distributors. |
| 2. Are critical spare parts such as collimators, handguards, and battery packs readily available for the Nomad system in 2026? | Yes. As of 2026, Aribex Inc. maintains an extensive inventory of OEM-certified spare parts, including tungsten collimators, medical-grade silicone handguards, battery packs, and protective lead sleeves. Distributors can access tiered bulk pricing and regional warehousing support. Aribex guarantees parts availability for a minimum of 7 years post-discontinuation of any model, ensuring long-term serviceability for clinics and resale value for distributors. |
| 3. What does the installation and commissioning process involve for the Nomad handheld X-ray unit? | The Nomad requires no structural installation or room shielding due to its ultra-low dose emissions and portable design. Commissioning includes on-site or virtual training by a certified technician covering radiation safety protocols, device calibration, Bluetooth connectivity to imaging software (DICOM 3.0 compliant), and integration with practice management systems. Distributors receive access to a digital onboarding portal with training modules, compliance checklists, and regulatory documentation for local health authority submissions. |
| 4. What warranty coverage is provided for the Nomad Pro 2™ in 2026, and are extended service plans available? | The Nomad Pro 2™ comes with a standard 3-year comprehensive warranty covering the X-ray tube, control board, battery, and housing against manufacturing defects. Extended warranty options are available for up to 5 years, including accidental damage protection and priority technical support. Distributors may offer white-glove service packages featuring loaner unit availability during repairs and guaranteed 72-hour turnaround for authorized service centers in North America, EU, and APAC regions. |
| 5. How does Aribex support regulatory compliance and technical updates for the Nomad system in 2026? | Aribex provides ongoing regulatory support including FDA 510(k), CE Marking (MDD/IVDR), and local country registrations (e.g., Health Canada, TGA, NMPA). Firmware updates are delivered securely via encrypted USB or cloud-based portals to enhance image optimization, dose control, and cybersecurity. All updates are validated to maintain compliance with IEC 60601-1 and ISO 13485 standards. Distributors receive quarterly technical bulletins and access to a dedicated compliance liaison team. |
Need a Quote for Nomad Handheld X Ray Machine?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


