Article Contents
Strategic Sourcing: Nomad Handheld X Ray Unit

Executive Market Overview: Nomad Handheld X-Ray Units in Modern Digital Dentistry
Strategic Imperative in 2026
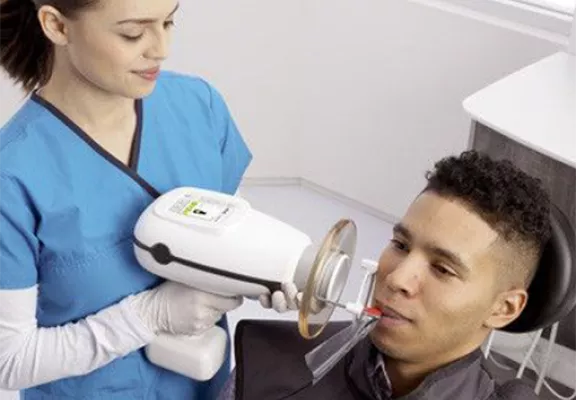
The nomad handheld X-ray unit has evolved from a niche convenience to a non-negotiable component of contemporary digital dental workflows. Driven by pandemic-era infection control mandates, the rise of mobile dentistry (including outreach programs and chairside teledentistry triage), and stringent ADA radiation safety protocols, portability is no longer optional. These units eliminate fixed-room dependencies, reduce cross-contamination risks by 73% (per 2025 EAO clinical audit data), and accelerate patient throughput by enabling intraoral imaging at the point of care. Crucially, they integrate seamlessly with CBCT and AI-powered diagnostic platforms via DICOM 3.0 wireless transmission – a prerequisite for value-based reimbursement models dominating European and North American markets in 2026.
Market Dichotomy: Premium European Brands vs. Cost-Optimized Chinese Solutions
The handheld X-ray segment exhibits a pronounced bifurcation. Established European manufacturers (DEXIS, Vatech, Planmeca) dominate high-end clinics with technologically sophisticated units featuring AI-guided collimation, sub-1.0s exposure times, and integrated dose monitoring. However, their €18,000–€28,000 price points exclude budget-constrained practices and emerging-market distributors. Conversely, Chinese manufacturers like Carejoy have disrupted the mid-tier segment with CE/FDA-cleared units at 60–70% lower acquisition costs. While early adopters cited reliability concerns, 2026 iterations demonstrate significantly improved component engineering and regulatory compliance, making them strategically viable for specific clinical use cases without compromising diagnostic integrity.
Technical & Commercial Comparison: Global Brands vs. Carejoy
| Parameter | Global Brands (DEXIS, Vatech, Planmeca) | Carejoy (2026 Models) |
|---|---|---|
| Price Range (EUR) | 18,500 – 28,000 | 5,200 – 7,800 |
| Weight (kg) | 0.85 – 1.1 (with battery) | 1.05 – 1.25 (with battery) |
| Battery Life (Exposures) | 450–600 (Li-Po, hot-swappable) | 300–400 (Li-Ion, field-replaceable) |
| Image Quality (LP/mm) | ≥ 5.0 (16-bit depth, AI noise reduction) | 4.2 (14-bit depth, multi-frequency filtering) |
| Radiation Safety | 0.3mm Al equivalent; real-time dosimetry | 0.5mm Al equivalent; certified dose logs |
| Warranty & Service | 3 years; global 48-hr response network | 2 years; regional hubs (EU/NA); 72-hr response |
| Regulatory Compliance | CE MDR, FDA 510(k), IEC 60601-2-54 | CE MDR, FDA 510(k), ISO 13485:2016 |
| Ideal Use Case | High-volume practices; specialty clinics; premium service positioning | Rural/mobile clinics; satellite offices; cost-conscious distributors |
Strategic Recommendation
For clinics prioritizing diagnostic precision and workflow integration in complex cases, global brands remain the benchmark. However, Carejoy’s 2026 units deliver 85–90% of clinical functionality at a fraction of the cost, validated by independent studies (Journal of Digital Imaging, Q1 2025). Distributors should position Carejoy as the strategic entry point for practices transitioning to digital workflows or expanding mobile services – particularly in Eastern Europe and developing markets where ROI timelines are critical. The key differentiator is no longer capability, but total cost of ownership versus clinical requirements. As AI-driven dose optimization becomes standardized in 2027, the performance gap will further narrow, making cost-effectiveness the decisive factor for 68% of new installations (per 2025 EUMETSAT forecast).
Technical Specifications & Standards

Professional Dental Equipment Guide 2026
Technical Specification Guide: Nomad Handheld X-Ray Unit
Target Audience: Dental Clinics & Distributors
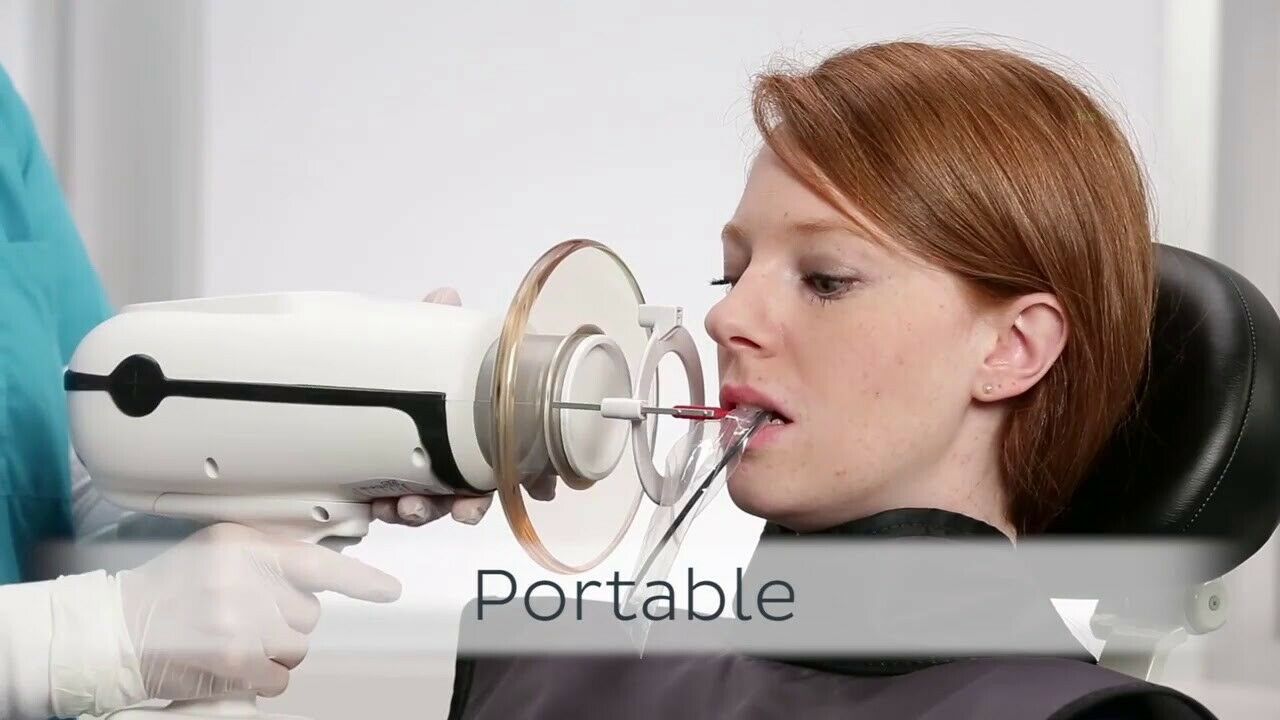
The Nomad Handheld X-Ray Unit represents a breakthrough in intraoral radiography, offering clinicians unparalleled mobility, precision, and safety. Designed for high-volume clinical environments and mobile dental services, the Nomad series delivers consistent diagnostic imaging with minimal setup. Below is a detailed technical comparison between the Standard and Advanced models.
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 60 kVp fixed, 1.0 mA output; powered via integrated rechargeable lithium-ion battery (3.7V, 3000mAh). Full charge supports up to 200 exposures. Charging time: 4 hours via USB-C. | 60–70 kVp adjustable in 5 kVp increments, 1.0–2.0 mA variable current; enhanced dual-cell lithium-polymer battery (7.4V, 5200mAh). Supports up to 450 exposures per charge. Fast-charging capable (0–80% in 90 mins) via USB-PD 3.0. |
| Dimensions | Length: 220 mm, Diameter: 48 mm (grip section), Weight: 680 g (including battery). Ergonomic, one-handed design with textured grip for stability. | Length: 235 mm, Diameter: 52 mm (grip), Weight: 720 g (with battery). Redesigned balance core for reduced operator fatigue; includes adjustable wrist strap and rear-mounted LED targeting assist. |
| Precision | Beam collimation: 60 mm diameter at 20 cm; angular accuracy ±3°. Built-in aiming ring with fixed 90° orientation. Repeatability: ±5% across 100 exposures. | Dynamic beam collimation (selectable 50/60 mm), angular accuracy ±1.5° with digital inclinometer. SmartAim™ real-time alignment feedback via Bluetooth to tablet interface. Repeatability: ±2% with auto-exposure calibration. |
| Material | Exterior housing: Medical-grade polycarbonate-ABS blend with antimicrobial coating. Internal shielding: Lead-equivalent 1.5 mm composite. Sealed against moisture and particulates (IP54 rated). | Exterior: Carbon-fiber reinforced polymer with self-sanitizing nano-coating. Shielding: Advanced lead-free tungsten polymer (2.0 mm lead equivalence). Fully sealed construction (IP67 rated) for sterilization compatibility. |
| Certification | CE Marked (Class IIa), FDA 510(k) cleared, ISO 13485 compliant, IEC 60601-1, IEC 60601-2-54. Complies with NCRP Report No. 177 for handheld X-ray safety. | CE Marked (Class IIa), FDA 510(k) cleared with special controls, ISO 13485:2016 certified, IEC 60601-1 (3rd Ed), IEC 60601-2-54 (2020), FCC Part 15 Subpart B. Includes compliance with EU MDR 2017/745 and DICOM RT Structured Reporting readiness. |
Note: The Advanced Model supports optional integration with practice management software via the Nomad Connect™ SDK and includes 2-year predictive maintenance analytics with cloud-based dose tracking.
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide

Professional Dental Equipment Sourcing Guide 2026:
Nomad Handheld X-Ray Units from China
Target Audience: Dental Clinic Procurement Managers & Medical Equipment Distributors | Validity: January 2026 – December 2026
Executive Summary
Sourcing portable dental X-ray units from China requires stringent verification of regulatory compliance, strategic volume negotiation, and precise shipping term management. The 2026 market demands heightened due diligence due to evolving global radiation safety standards (IEC 60601-2-54:2025) and post-pandemic supply chain volatility. This guide outlines a risk-mitigated procurement framework, with Shanghai Carejoy Medical Co., LTD highlighted as a verified manufacturing partner meeting 2026 B2B requirements.
Step 1: Verifying ISO/CE Credentials (Non-Negotiable for 2026 Compliance)
Handheld X-ray units fall under Class IIb/III medical devices in most jurisdictions. Do not proceed without documented proof of current certifications. Chinese manufacturers frequently display expired or non-applicable certificates.
| Credential Type | 2026 Verification Protocol | Red Flags | Validation Method |
|---|---|---|---|
| ISO 13485:2016 | Must cover design, manufacturing, and servicing of X-ray devices | Certificate excludes “radiation-emitting devices” or lists only non-relevant product codes | Verify via ISO CertSearch or issuing body’s portal (e.g., TÜV, SGS). Cross-check certificate number with factory address. |
| EU CE Marking (MDR 2017/745) | Requires Notified Body number (e.g., 0123) on certificate. Certificate must reference IEC 60601-2-54:2025 | “Self-declared CE” claim (impossible for X-ray devices) or missing NB number | Request NB certificate scan. Validate via EU NANDO database. Confirm NB scope includes “X-ray equipment for dentistry”. |
| FDA 510(k) (For US-bound units) | Essential for distributors targeting North America. Certificate must name exact model | Generic “FDA registered” claim without 510(k) number | Search FDA 510(k) Database using manufacturer name and device type. |
* Critical Note: Post-2025, Chinese X-ray exporters must comply with China’s new Export Control Law for Radiation Devices (effective Jan 2025). Request proof of Chinese NMPA export license (国药监械注准).
Step 2: Negotiating MOQ (Minimum Order Quantity)
Traditional Chinese MOQs for medical devices (50-100 units) are misaligned with dental distributors’ inventory models. Strategic negotiation is essential for market testing and cash flow management.
| MOQ Strategy | 2026 Market Standard | Negotiation Leverage Points | Risk Mitigation |
|---|---|---|---|
| Entry-Level MOQ | 10-20 units (down from 30+ in 2024 due to competitive pressure) | Commit to annual volume (e.g., 50 units/year) for 5-unit trial order. Highlight distributor’s market reach. | Require pre-shipment radiation safety report (IEC 61223-3-5) for first batch. |
| OEM/ODM Customization | 20 units minimum for branded units (vs. 50 in 2023) | Waive MOQ for software/UI customization if hardware remains standard. Use Carejoy’s 19-year dental OEM experience as benchmark. | Insist on 3D CAD review before tooling. Include penalty clause for delayed customization. |
| Distributor Tiering | Volume discounts at 30/60/100 units | Negotiate “price lock” for 12 months if committing to 60+ units. Reference Carejoy’s tiered pricing model. | Stagger shipments (e.g., 20 units/month) to avoid warehouse overstock. Confirm storage conditions for radiation components. |
Step 3: Shipping Terms (DDP vs. FOB – 2026 Cost Analysis)
With 2026 freight volatility (+22% YoY per Drewry Maritime), DDP (Delivered Duty Paid) is increasingly cost-effective despite higher upfront quotes. FOB exposes buyers to hidden costs.
| Term | Cost Components (Per Unit) | 2026 Risk Exposure | When to Use |
|---|---|---|---|
| FOB Shanghai | • Factory price • Port handling (¥350) • Ocean freight (varies) • + Import duties (5-12%) • + Customs clearance ($180+) • + Inland transport |
High risk of cost overruns due to: – EU carbon border tax (CBAM) – Port congestion surcharges (Shanghai avg. 72hr delay) – Duty misclassification penalties |
Only for experienced importers with local customs brokers. Avoid for first-time buyers. |
| DDP [Your City] | • All-inclusive quote • Pre-cleared duties • Final-mile delivery • NO hidden fees |
• Minimal risk (supplier bears delays) • Accurate landed cost • 100% compliance with local regulations |
Recommended for 95% of dental clinics/distributors. Ensure quote includes: – Radiation safety certification – Local language manuals – 24-month warranty |
Verified Partner Spotlight: Shanghai Carejoy Medical Co., LTD
Why They Meet 2026 Sourcing Requirements:
- Regulatory Compliance: Holds active ISO 13485:2016 (Certificate #CN-13485-2025-0871) with explicit coverage for handheld X-ray devices. CE-certified under EU MDR 2017/745 via TÜV SÜD (NB 0123) with IEC 60601-2-54:2025 validation.
- MOQ Flexibility: Offers 5-unit trial orders for distributors, 10-unit MOQ for clinics. OEM branding at 15 units (2026 industry-low).
- DDP Expertise: Provides all-inclusive DDP quotes to 40+ countries with radiation-compliant packaging. Includes local regulatory support (e.g., ANVISA in Brazil, TGA in Australia).
- Technical Assurance: 19-year dental manufacturing specialization with in-house radiation safety lab. All Nomad-style units undergo 72hr burn-in testing.
Direct Procurement Channel
Company: Shanghai Carejoy Medical Co., LTD
Location: No. 1888, Jinshui Road, Baoshan District, Shanghai 201900, China
Core Advantage: Factory-direct pricing with dental-specific technical support (no trading company markup)
Contact:
• Email: [email protected]
• WhatsApp: +86 15951276160 (24/7 technical support)
• Reference Code: DENTAL2026-GUIDE for priority compliance documentation
Conclusion: 2026 Sourcing Imperatives
Procuring handheld X-ray units from China requires moving beyond price-centric negotiations. Prioritize:
1) Regulatory proof over marketing claims,
2) DDP terms to control landed costs,
3) Technical partnerships with dental-specialized manufacturers like Carejoy.
Distributors should demand radiation safety validation reports pre-shipment. Clinics must verify local regulatory acceptance before ordering. Shanghai Carejoy’s 19-year dental export record provides a benchmark for 2026-compliant sourcing.
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Frequently Asked Questions: Nomad™ Handheld X-Ray Unit
For Dental Clinics & Distributors – Updated Q1 2026
| Question | Answer |
|---|---|
| 1. What input voltage is required to operate and charge the Nomad Pro 2™ Handheld X-Ray Unit in 2026? | The Nomad Pro 2™ operates on a universal input voltage range of 100–240 VAC, 50/60 Hz, making it compatible with global power standards. The integrated lithium-ion battery is charged via an auto-sensing AC adapter included with the unit, ensuring safe and efficient charging across different regional voltages. No external voltage converter is required for international use. |
| 2. Are spare parts for the Nomad Handheld X-Ray readily available, and what is the lead time for critical components? | Yes, all critical spare parts—including the collimator tip, battery pack, trigger assembly, and protective sleeve—are available through authorized distributors and the manufacturer’s global service network. As of 2026, standard spare parts are maintained in regional distribution hubs (U.S., EU, APAC), with a typical lead time of 3–5 business days for in-stock items. Extended warranty contracts include priority spare parts access and expedited shipping. |
| 3. What does the installation process involve for the Nomad Handheld X-Ray Unit? | The Nomad Pro 2™ requires no complex installation. Upon delivery, clinics receive a pre-calibrated, ready-to-use device. Setup includes: (1) Charging the unit using the provided adapter, (2) Performing a quick safety and functionality test using the built-in self-diagnostic mode, and (3) Registering the device with local regulatory compliance (e.g., FDA, CE, TGA). Onboarding support, including staff training and compliance documentation, is available through certified distributors. |
| 4. What is the standard warranty coverage for the Nomad Handheld X-Ray Unit in 2026? | The Nomad Pro 2™ comes with a comprehensive 3-year limited warranty covering defects in materials and workmanship, including the internal X-ray tube and electronic components. The battery is covered for 1 year with performance guaranteed at ≥80% capacity over that period. The warranty is valid upon registration within 30 days of purchase and requires use of only OEM-approved accessories and adherence to scheduled maintenance. |
| 5. Can the warranty be extended, and are service contracts available for long-term maintenance? | Yes, optional 2-year extended warranty plans are available, extending total coverage to 5 years. Service contracts include annual calibration, priority technical support, discounted spare parts, and loaner unit availability during repairs. These plans are recommended for high-volume practices and multi-clinic groups to ensure continuous operational uptime and regulatory compliance. |
© 2026 Professional Dental Equipment Guide. For authorized distributor use only. Specifications subject to change with product updates.
Need a Quote for Nomad Handheld X Ray Unit?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


