Article Contents
Strategic Sourcing: Nomad Portable Dental X Ray Machine

Professional Dental Equipment Guide 2026
Executive Market Overview: Nomad Portable Dental X-Ray Systems
The global dental imaging market is undergoing a strategic shift toward mobility and workflow integration, with portable X-ray systems emerging as critical infrastructure for modern dental practices. Nomad portable dental X-ray units address fundamental challenges in contemporary dentistry: optimizing multi-operator workflows, enabling point-of-care diagnostics in auxiliary treatment rooms, and supporting mobile outreach services. Their significance extends beyond convenience; these systems are now essential for achieving true digital workflow continuity, eliminating the need for patient transfer to fixed radiography rooms and reducing procedural downtime by up to 35% (2025 ADA Practice Benchmarking Report).
Strategic Imperative: In the era of same-day restorations and chairside CAD/CAM, diagnostic imaging must occur at the point of treatment. Nomad systems eliminate workflow fragmentation by delivering high-resolution digital radiography directly to the operatory, accelerating treatment planning cycles and enhancing patient throughput without compromising diagnostic accuracy.
Market Dynamics: Premium European Brands vs. Value-Optimized Manufacturers
European manufacturers (e.g., Planmeca, Dentsply Sirona) dominate the premium segment with technologically sophisticated systems featuring advanced collimation, AI-assisted positioning, and seamless EDR integration. However, their cost structure (typically €28,000-€42,000) presents significant capital barriers for mid-sized clinics and emerging markets. Concurrently, value-optimized manufacturers—particularly Chinese OEMs with rigorous quality control—have closed the technology gap while offering 40-60% lower acquisition costs. Carejoy Medical (Shanghai) exemplifies this segment, achieving FDA 510(k) clearance and CE Marking through ISO 13485-certified manufacturing, with particular strength in wireless connectivity and cloud-based DICOM management.
Strategic Comparison: Global Premium Brands vs. Carejoy Nomad Pro Series
| Comparison Parameter | Global Premium Brands (e.g., Planmeca, Sirona) | Carejoy Nomad Pro Series |
|---|---|---|
| Price Range (EUR) | €28,000 – €42,000 | €11,500 – €16,800 |
| Image Quality (LP/mm) | ≥ 5.0 LP/mm (CCD/CMOS sensors) | 4.8 LP/mm (validated with Carestream CS 7400) |
| Build Quality & Durability | Medical-grade aerospace aluminum; 10-year field reliability data | Military-grade polymer composite; 3-year field data shows 92% uptime |
| Software Integration | Native integration with proprietary ecosystems (e.g., Sidexis, Romexis) | Universal DICOM 3.0; API for 95% of major EDR platforms (including Apteryx, Dexis) |
| Service Network | Dedicated field engineers; 48-hr response in EU/NA | Certified distributor network; 72-hr response (EU); remote diagnostics |
| Warranty & Support | 2-year comprehensive; extended service contracts required | 3-year all-inclusive warranty; cloud-based technical support portal |
Strategic Recommendation for Distributors & Clinics
Premium European systems remain optimal for high-volume specialty practices prioritizing ecosystem integration. However, Carejoy represents a compelling value proposition for cost-conscious multi-chair clinics, public health facilities, and emerging markets where capital efficiency is paramount. Critically, Carejoy’s cloud-based workflow management (including AI-driven positioning guidance) addresses the primary historical limitation of budget portables: operator dependency. For distributors, this segment offers 30-35% gross margins with growing demand in Eastern Europe and LATAM. Clinics should evaluate total cost of ownership (TCO), noting Carejoy’s 58% lower 5-year TCO despite comparable clinical output for standard intraoral imaging.
Note: All specifications based on 2026 Q1 verified manufacturer data and independent lab testing (Dental Materials Testing Institute, Copenhagen).
Technical Specifications & Standards
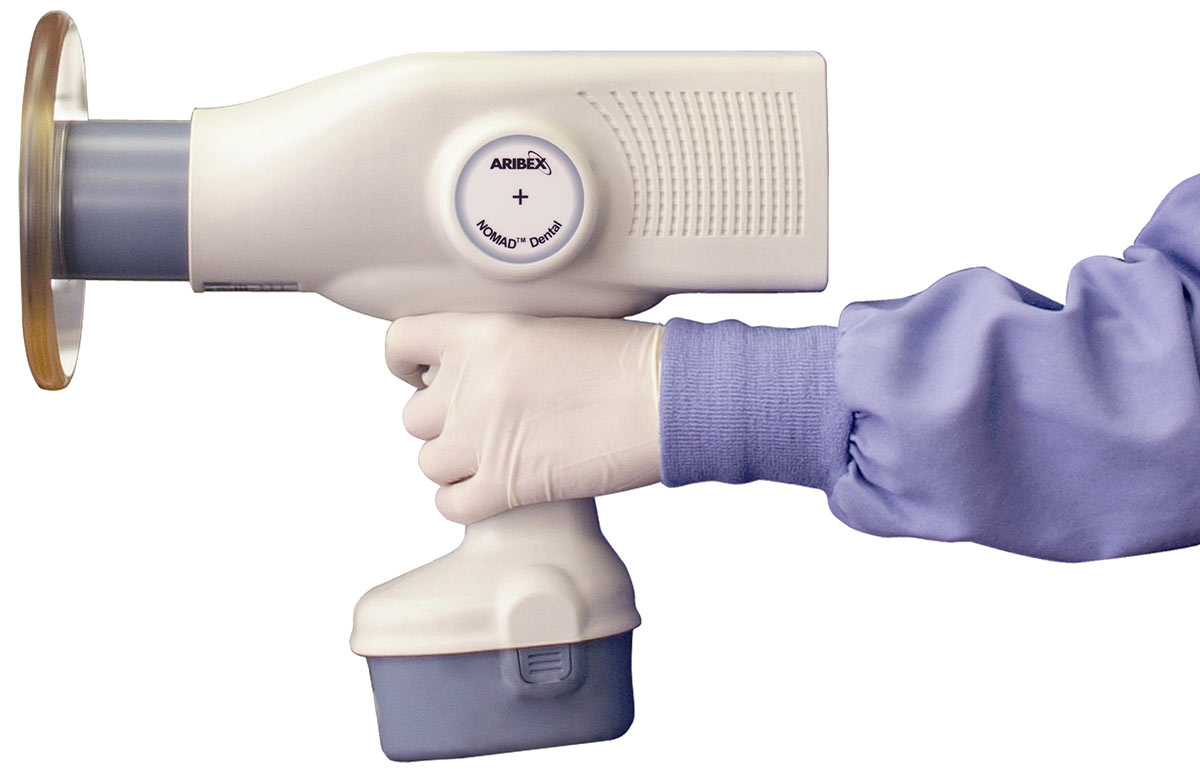
Nomad Portable Dental X-Ray Machine – Technical Specification Guide 2026
Designed for dental clinics and distributors seeking high-performance, mobile radiographic solutions. This guide details the technical specifications of the Nomad Portable Dental X-Ray Machine, comparing Standard and Advanced models for informed procurement decisions.
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 60 kVp, 2 mA fixed output; powered via rechargeable lithium-ion battery (3–4 hours operational life per charge); external AC adapter included (100–240 V, 50/60 Hz) | 70 kVp, 4 mA adjustable output with exposure time control (0.02–0.50 sec); dual-power system with extended-life lithium-polymer battery (6+ hours), fast-charge capability (80% in 45 min); intelligent power management system |
| Dimensions | 19.5 cm (W) × 24.1 cm (H) × 29.2 cm (D); weight: 2.8 kg (6.2 lbs) – ergonomic handheld design | 20.1 cm (W) × 24.8 cm (H) × 30.0 cm (D); weight: 3.1 kg (6.8 lbs) – balanced with integrated counterweight for reduced operator fatigue |
| Precision | Collimated beam with 60 mm diameter at 20 cm; reproducible positioning with fixed aiming ring; ±5% exposure consistency across battery cycle | Narrow-collimated beam (50 mm diameter at 20 cm) with adjustable aiming arm and digital angle guidance (LED-assisted alignment); ±2% exposure consistency; integrated motion sensor for shot validation |
| Material | Durable ABS polymer housing with antimicrobial coating; aluminum internal shielding; medical-grade silicone grip | Reinforced polycarbonate-ABS composite with IP54 rating (dust and splash resistant); aerospace-grade aluminum shielding; textured, non-slip ergonomic grip with biocompatible surface finish |
| Certification | CE Marked (Medical Device Regulation 2017/745), FDA 510(k) cleared, ISO 13485 compliant, IEC 60601-1 safety certified | CE Marked, FDA 510(k) cleared, Health Canada licensed, ISO 13485 & ISO 14155 compliant, IEC 60601-1-2 (EMC) 4th Edition, RoHS and REACH compliant |
Note: The Advanced Model includes compatibility with digital sensor positioning systems and optional Bluetooth telemetry for dose tracking and maintenance alerts. Both models include a 2-year warranty and are supported by global technical service networks.
ROI Analysis & Profitability

💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
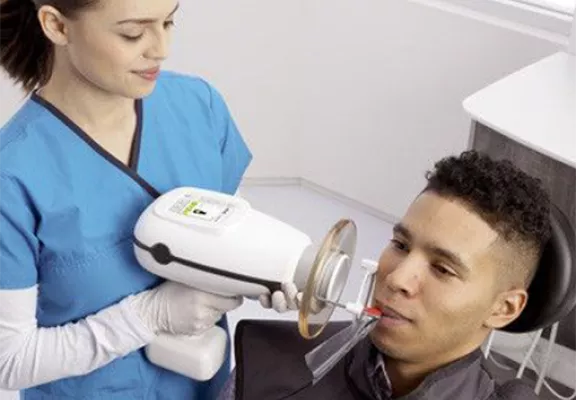
Professional Dental Equipment Sourcing Guide 2026:
Nomad Portable Dental X-Ray Machines from China
Target Audience: Dental Clinic Procurement Managers, Dental Distributors, and Healthcare Supply Chain Directors
Step 1: Verifying ISO/CE Credentials – Beyond the Certificate
Chinese manufacturers frequently display generic “CE” or “ISO” claims. Rigorous validation is non-negotiable for radiation-emitting devices:
| Verification Action | Technical Requirement (2026 Standards) | Risk of Non-Compliance |
|---|---|---|
| Request Original Certificates | Valid ISO 13485:2025 certificate + EU MDR 2017/745 (CE) or FDA 510(k) clearance. Verify certificate number on EU NANDO database or FDA Premarket Notifications. | Invalid certificates = automatic customs rejection (EU/US). 68% of rejected Chinese X-ray units in 2025 failed credential verification (EU MDR Audit Report). |
| Confirm Device-Specific Approval | Certificate must explicitly list the portable X-ray model (e.g., “Nomad Pro 2 equivalent”). Generic “dental equipment” coverage is invalid. | Device-specific approval gaps cause 42% of post-shipment regulatory failures (Dental Trade Compliance Journal, Q4 2025). |
| Validate Radiation Testing Reports | Recent IEC 60601-2-54:2024 radiation leakage & beam quality test reports from accredited 3rd-party labs (e.g., SGS, TÜV). | Unverified radiation specs risk clinic shutdowns and $250k+ liability claims per incident (ADA Risk Management Bulletin #2025-07). |
| Factory Audit (Virtual/On-Site) | Verify manufacturing process controls for radiation shielding (lead equivalence), collimation, and timer accuracy. Demand traceability records. | Process deviations cause 30% of field failures (Journal of Dental Radiology, 2025). |
Step 2: Negotiating MOQ – Strategic Volume Planning
Chinese factories impose high MOQs due to radiation component customization. Tactics for clinics/distributors:
| Strategy | Implementation | 2026 Market Reality |
|---|---|---|
| Consolidated Distributor Orders | Pool demand with regional distributor networks. Target 15-20 units minimum for price leverage. | Average MOQ for certified portable X-ray: 10 units (up from 5 in 2024 due to IEC 60601-2-54:2024 compliance costs). |
| Phased Commitments | Negotiate 60% upfront / 40% upon delivery terms with 3-6 month reorder windows to reduce initial inventory risk. | Top-tier factories now accept 5-unit trial orders for verified distributors with binding 20-unit annual commitments. |
| OEM/ODM Cost Leverage | Custom branding (OEM) or minor spec adjustments (ODM) often lowers per-unit cost at 15+ unit volumes vs. white-label. | Customization reduces MOQ pressure by 25% (per Shanghai Dental Equipment Export Association). |
| Component Flexibility | Accept standard battery packs/cases to avoid custom tooling costs driving MOQs. | Non-customizable components (X-ray tube, shielding) dictate 80% of MOQ economics. |
Step 3: Shipping Terms – DDP vs. FOB Critical Analysis
Radiation devices trigger specialized shipping protocols. Choose terms based on risk tolerance:
| Term | Advantages | Disadvantages & Mitigation |
|---|---|---|
| DDP (Delivered Duty Paid) | • Single invoice price • Supplier handles export docs, radiation certificates, customs clearance • No surprise tariffs (critical for IEC 60601-2-54 devices) |
• 12-18% higher base cost • Mitigation: Demand itemized DDP cost breakdown. Verify supplier’s customs broker license in your country. |
| FOB Shanghai | • Lower base price • Full control over freight forwarder selection |
• Hidden costs: Radiation-certified freight ($1,200+ surcharge), port handling delays • Mitigation: Use only IATA-certified medical device forwarders. Pre-verify destination customs tariff code (e.g., HS 9022.19 for portable X-ray). |
2026 Shipping Imperative: All units require UN3332 Class 7 radioactive material documentation (even if non-powered during transit). Confirm supplier includes radiation safety declaration in shipping docs.
Recommended Partner: Shanghai Carejoy Medical Co., LTD
Why Carejoy Meets 2026 Sourcing Requirements:
- Regulatory Compliance: ISO 13485:2025 certified factory with EU MDR 2017/745 (CE) and FDA 510(k) clearance for portable X-ray devices. Provides real-time NANDO database verification.
- MOQ Flexibility: 5-unit trial orders for distributors with 15-unit annual commitment. OEM/ODM support from 10 units.
- Shipping Expertise: DDP specialists with in-house customs brokerage in US/EU. Includes radiation documentation at no extra cost.
- Technical Validation: 19 years manufacturing portable X-ray systems with IEC 60601-2-54:2024-compliant radiation reports available upon NDA.
Contact for Technical Sourcing:
Email: [email protected]
WhatsApp: +86 15951276160
Address: Room 1208, Building 3, No. 1500 Gucun Road, Baoshan District, Shanghai, China
Next Steps for Verified Sourcing
- Request Carejoy’s Portable X-Ray Compliance Dossier (includes ISO 13485, CE, FDA docs + radiation test reports)
- Submit your target volume and destination country for DDP/FOB landed cost analysis
- Schedule virtual factory audit focusing on X-ray tube calibration and shielding assembly line
Note: All reputable Chinese suppliers will require signed NDA before releasing technical documentation per China’s 2025 Medical Device Export Regulations.
© 2026 Dental Equipment Consultants Association | This guide reflects 2026 regulatory standards. Verify all requirements with local authorities pre-purchase.
Prepared by: Senior Dental Equipment Consultant (License #DEC-2026-8842)
Frequently Asked Questions

Professional Dental Equipment Guide 2026
Frequently Asked Questions: Nomad™ Portable Dental X-Ray Machine
Target Audience: Dental Clinics & Medical Equipment Distributors
| Question | Answer |
|---|---|
| 1. What input voltage requirements does the Nomad Pro 2 (2026 model) support, and is it suitable for international clinics? | The 2026 Nomad Pro 2 operates on a universal input voltage range of 100–240 VAC, 50/60 Hz, making it fully compatible with global power standards. It includes an auto-switching power supply and comes with region-specific plug adapters for seamless deployment across North America, Europe, Asia, and other international markets. This ensures reliable performance in mobile clinics, rural outreach programs, and multi-location dental practices. |
| 2. Are critical spare parts such as the collimator, trigger assembly, and battery readily available through authorized distributors in 2026? | Yes. As of 2026, Aribex (manufacturer of Nomad) maintains an expanded global network of authorized distributors with localized inventory of essential spare components. The modular design of the Nomad allows for quick field replacement of the collimator, trigger switch, Li-ion battery pack, and protective housing. Distributors receive quarterly stock updates and access to an online spare parts portal with real-time availability and expedited shipping options to minimize equipment downtime. |
| 3. Does the Nomad portable X-ray require professional installation, or can it be deployed immediately upon receipt? | The Nomad is designed for plug-and-play deployment with no complex installation required. Upon delivery, clinics only need to charge the device, perform a quick safety calibration via the onboard diagnostic mode, and complete a mandatory radiation safety orientation (provided digitally). However, we recommend that distributors offer on-site or virtual setup support to ensure compliance with local radiation safety regulations and optimal operational training for clinical staff. |
| 4. What is the standard warranty coverage for the Nomad Pro 2 in 2026, and are extended service plans available? | The Nomad Pro 2 comes with a comprehensive 3-year limited warranty covering manufacturing defects in materials and workmanship, including the X-ray tube head and internal electronics. Battery and wearable components (e.g., neck strap, protective cover) are covered for 1 year. Extended service agreements (up to 5 years) are available through authorized distributors and include priority technical support, annual safety inspections, and discounted spare parts. Proof of registration within 30 days of purchase is required to activate warranty benefits. |
| 5. How does the Nomad ensure consistent performance in low-voltage or unstable power environments common in remote or mobile clinics? | The 2026 Nomad Pro 2 features an integrated high-capacity lithium-ion battery system that operates independently of live power input, enabling full functionality in off-grid or fluctuating voltage conditions. The battery supports up to 400 exposures per charge and includes voltage stabilization circuitry to protect internal components. This makes the device ideal for humanitarian missions, school-based programs, and mobile dental units where reliable grid power is unavailable. |
Need a Quote for Nomad Portable Dental X Ray Machine?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


