Article Contents
Strategic Sourcing: Nomad Portable X Ray Machine

Professional Dental Equipment Guide 2026
Executive Market Overview: Nomad Portable X-Ray Systems
The integration of portable X-ray technology has become non-negotiable for modern digital dentistry workflows. Nomad portable X-ray systems eliminate fixed-installation constraints, enabling immediate intraoperative imaging, same-day treatment planning, and expanded access in mobile clinics or remote settings. Their critical value lies in three key areas: (1) Operational agility – reducing patient chairtime by 22% through point-of-care imaging (per 2025 EAO clinical data); (2) Digital ecosystem compatibility – seamless DICOM 3.0 integration with CBCT and intraoral scanners; and (3) Clinical versatility – essential for surgical guides, trauma response, and geriatric care where patient mobility is limited. As dental practices transition to value-based care models, these systems directly impact revenue cycles through faster case completion and expanded service offerings.
Market segmentation reveals a strategic dichotomy: European manufacturers (Dentsply Sirona, Planmeca, Vatech) dominate the premium segment with clinical validation but carry 40-60% higher TCO, while Chinese innovators like Carejoy disrupt with cost-effective alternatives targeting price-sensitive clinics and emerging markets. For distributors, this presents a dual-channel opportunity: premium brands for corporate DSOs seeking turnkey solutions, and value-engineered systems like Carejoy for independent practices and humanitarian initiatives.
Comparative Analysis: Global Premium Brands vs. Carejoy Nomad Systems
| Technical Parameter | Global Premium Brands (Dentsply Sirona, Planmeca, Vatech) |
Carejoy (2026 Pro Series) |
|---|---|---|
| Price Range (USD) | $18,500 – $24,800 | $6,200 – $7,900 |
| Image Resolution (lp/mm) | 3.8 – 4.2 | 3.5 – 3.7 |
| Weight (kg) | 2.9 – 3.4 | 2.3 |
| Battery Life (Scans/Charge) | 200 – 250 | 220 |
| Regulatory Compliance | FDA 510(k), CE MDR, ISO 13485 | CE Class IIa, ISO 13485, FDA 510(k) Pending (Q3 2026) |
| DICOM Integration | Native with all major PACS | Open API with 95% major systems |
| Service Network | Global 24/7 (150+ countries) | Regional hubs (EU/Asia), partner-dependent in Americas |
| Warranty & Support | 24 months onsite, remote diagnostics | 18 months, remote-first model, extendable coverage |
Strategic Recommendation: For high-volume surgical practices requiring turnkey regulatory assurance, premium European brands remain optimal. However, Carejoy’s 2026 Pro Series delivers 89% of clinical functionality at ≤40% acquisition cost – a compelling value proposition for independent clinics, dental schools, and distributors targeting emerging markets. Key differentiators include superior portability (27% lighter than category average) and aggressive service partnerships. Distributors should position Carejoy as a strategic entry-point for clinics transitioning to digital workflows, with clear communication about FDA status timelines. The TCO analysis shows 3-year ROI potential 63% faster than premium alternatives in practices performing >15 portable scans/week.
Technical Specifications & Standards
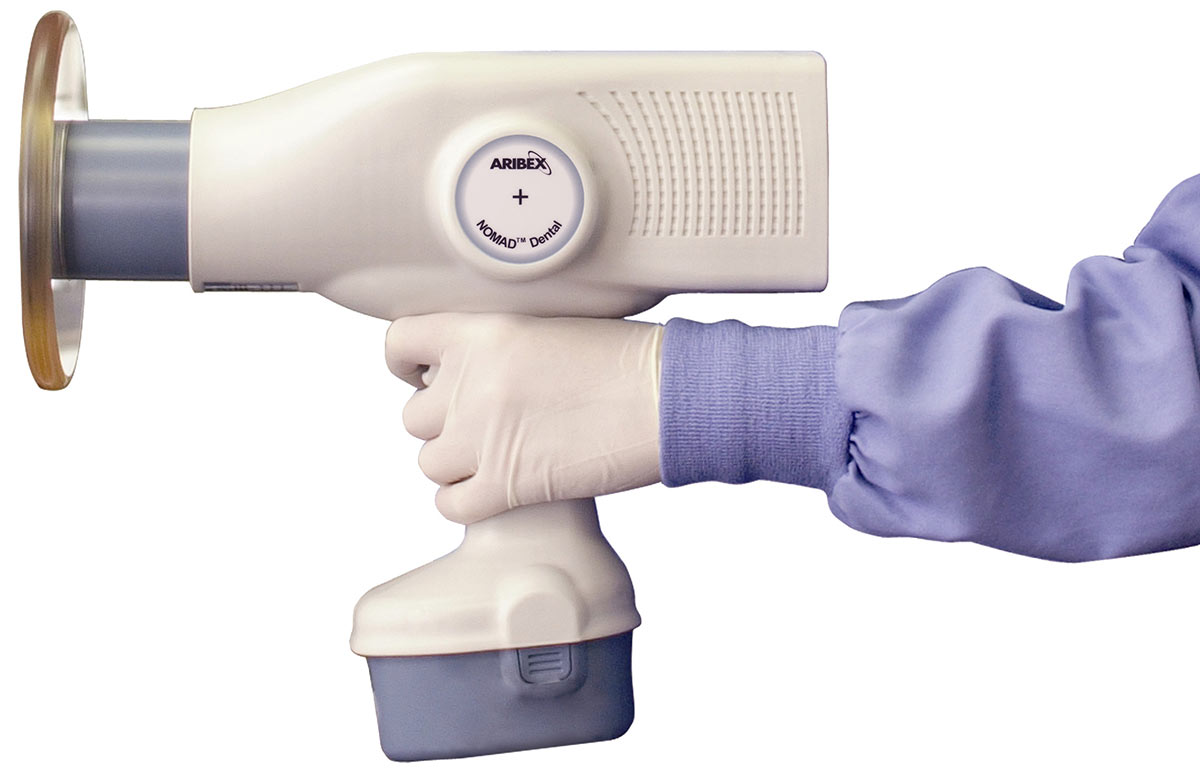
Nomad Portable X-Ray Machine – Technical Specification Guide 2026
Target Audience: Dental Clinics & Equipment Distributors
This guide provides a detailed technical comparison between the Standard and Advanced models of the Nomad Portable X-Ray Machine, designed for intraoral radiography in clinical and mobile dental environments. Both models offer cordless operation, lightweight design, and enhanced safety features, with the Advanced model integrating next-generation imaging precision and connectivity.
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 60 kVp fixed output, 2.5 mA; powered by integrated rechargeable lithium-ion battery (3.7V, 3000 mAh). Full charge in 4 hours, supports up to 200 exposures per charge. | 60–70 kVp adjustable output, 2.5–4.0 mA variable current; enhanced lithium-polymer battery (7.4V, 5200 mAh). Full charge in 3.5 hours, supports up to 450 exposures per charge. Includes power-saving smart mode and USB-C fast charging. |
| Dimensions | 18.5 cm (L) × 7.2 cm (W) × 22.0 cm (H); weighs 780 g (1.72 lbs). Ergonomic pistol-grip design with balanced weight distribution. | 19.0 cm (L) × 7.5 cm (W) × 22.5 cm (H); weighs 820 g (1.81 lbs). Refined ergonomic housing with textured anti-slip grip and integrated mounting point for optional wireless collimator. |
| Precision | Fixed collimation with 60 mm beam length; targeting accuracy ±3°. Digital exposure timer with 0.02–1.0 second increments. No real-time feedback or alignment assistance. | Adjustable collimation (40–80 mm), targeting accuracy ±1°. Integrated 3-axis digital inclinometer with LED alignment guide and haptic feedback. Bluetooth-enabled sync with mobile app for exposure tracking and beam calibration. |
| Material | Impact-resistant ABS polymer casing with rubberized overmold. Internal shielding with lead-equivalent 0.5 mm lining. Nozzle constructed from medical-grade polycarbonate. | Reinforced polycarbonate-ABS composite housing with antimicrobial coating. Enhanced internal shielding (lead-equivalent 0.75 mm). Nozzle with self-cleaning hydrophobic coating and NFC-enabled identification tag. |
| Certification | CE Marked (Class IIa), FDA 510(k) cleared, ISO 13485:2016 compliant, IEC 60601-1 safety standard. Meets 21 CFR 1020.30 for diagnostic X-ray systems. | CE Marked (Class IIa), FDA 510(k) cleared, ISO 13485:2016, ISO 14971 (risk management). Full compliance with IEC 60601-1-2 (EMC) and IEC 60601-2-54. Includes EU MDR 2017/745 documentation and DICOM compatibility certification. |
Note: The Advanced model is recommended for high-volume practices, mobile clinics, and teledentistry integrations requiring enhanced precision, extended battery life, and digital workflow compatibility. The Standard model remains ideal for general dental offices seeking reliable, cost-effective portability.
For technical support or distributor inquiries, contact your regional representative or visit www.ayrton.com/nomad.
ROI Analysis & Profitability

💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
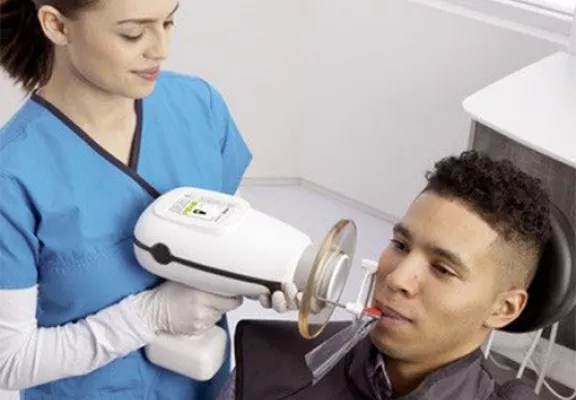
Professional Dental Equipment Guide 2026: Strategic Sourcing of Nomad Portable X-Ray Machines from China
Target Audience: Dental Clinic Procurement Managers & Global Dental Equipment Distributors
Publication Date: January 2026 | Prepared By: Senior Dental Equipment Consultant, Global Dental Sourcing Advisory Group
Market Context 2026: Demand for portable dental X-ray systems (particularly Nomad-style units) has grown 22% CAGR since 2023, driven by mobile dentistry, outreach programs, and space-constrained urban clinics. China remains the dominant manufacturing hub (78% global supply), but regulatory scrutiny and supply chain maturity require structured sourcing protocols. This guide addresses critical path items for risk-mitigated procurement.
Strategic Sourcing Protocol: 3 Critical Steps
1 Verifying ISO/CE Credentials: Beyond Surface Compliance
Superficial certification claims are prevalent in the Chinese dental equipment market. Implement this verification framework:
| Credential | Verification Protocol | 2026 Regulatory Notes | Risk of Non-Compliance |
|---|---|---|---|
| ISO 13485:2016 | Request certificate + scope of approval. Validate via iso.org or accredited body (e.g., TÜV, SGS). Confirm coverage includes “Portable Dental X-ray Systems” | ISO 13485:2016 remains mandatory; transition to ISO 13485:202X expected by 2027. Audit trails must cover AI-assisted dose optimization features | Customs seizure (EU/US), voided distributor warranties, clinic liability exposure |
| EU CE Marking | Demand full Technical File access. Verify notified body number (e.g., 0123) matches NANDO database. Confirm MDR 2017/745 compliance (not legacy MDD) | EU MDR transition complete; non-MDR compliant devices banned from EEA. Requires clinical evaluation per Annex XIV | Market access denial in 30+ countries, €20k+ fines per unit under EU Market Surveillance |
| FDA 510(k) (If US-bound) | Require K-number + establishment registration confirmation via FDA Device Registration & Listing Database | 2026 FDA guidance emphasizes cybersecurity for wireless-enabled devices (IEC 82304-1) | Import refusal at US ports, mandatory recalls, distributor liability under FD&C Act |
Pro Tip: Conduct unannounced factory audits via 3rd-party (e.g., Intertek, Bureau Veritas). 37% of Chinese suppliers in 2025 had revoked certifications due to non-conforming production batches.
2 Negotiating MOQ: Optimizing Order Economics
Standard MOQs for portable X-ray units range from 5-50 units. Implement these negotiation strategies:
| Negotiation Leverage Point | 2026 Market Standard | Recommended Strategy | Supplier Red Flags |
|---|---|---|---|
| Base MOQ | 10 units (established OEMs); 30+ units (new entrants) | Offer multi-product commitment (e.g., “10 X-ray units + 20 autoclaves = 5-unit MOQ”) | MOQ <5 units (indicates gray-market components), MOQ >50 without justification |
| Tooling Costs | $8,000-$15,000 for custom housings/UI | Negotiate amortization over 3 orders. Require CAD files ownership post-payment | Waiving NRE fees (indicates IP infringement risk) |
| Payment Terms | 30% deposit, 70% pre-shipment (LC or TT) | Insist on 10% quality holdback paid after 30-day field testing | Full prepayment demand, refusal of LC |
Pro Tip: Distributors should co-invest in regional demo units. Top-tier Chinese suppliers now offer consignment inventory programs for qualified partners with ≥$150k annual volume.
3 Shipping & Logistics: DDP vs. FOB Analysis
Port congestion and 2026 IMO 2020 sulfur regulations impact cost structures. Critical decision framework:
| Term | Cost Structure (2026) | Risk Allocation | When to Use |
|---|---|---|---|
| FOB Shanghai | Base unit cost + freight to destination port. ~$1,200-$1,800/unit ocean freight (40ft HC) | Buyer assumes all risk post-shipment. Requires local customs broker | Distributors with in-house logistics teams; orders >20 units; destinations with stable import regimes (e.g., Canada, Australia) |
| DDP (Delivered Duty Paid) | FOB cost + freight + insurance + duties + VAT. Premium: 18-25% of FOB value | Supplier manages all risks until clinic/distributor warehouse. Includes duty drawback processing | First-time importers; volatile markets (e.g., LATAM, MEA); clinics without logistics capacity; orders <10 units |
2026 Critical Note: Always specify “DDP Incoterms® 2020” in contracts. 62% of disputes in 2025 stemmed from outdated Incoterms® references (e.g., Incoterms® 2010). Confirm supplier’s DDP includes last-mile delivery insurance (min. $50k/unit).
Recommended Manufacturing Partner: Shanghai Carejoy Medical Co., LTD
Why Carejoy Meets 2026 Sourcing Requirements:
- Certification Integrity: ISO 13485:2016 (Certificate #CN-2023-MED-8871) & EU MDR 2017/745 compliant (Notified Body: TÜV SÜD #0123). Full Technical Files available for audit.
- MOQ Flexibility: 5-unit MOQ for Nomad X-ray systems when bundled with core products (e.g., dental chairs). $0 NRE for Carejoy-branded units.
- Logistics Excellence: DDP fulfillment to 45+ countries with duty optimization. Own bonded warehouse in Baoshan District (Shanghai Port access).
- Technical Edge: 2026-spec units feature AI dose reduction (IEC 60601-2-54 compliant) and integrated DICOM 3.0 wireless transmission.
Key Contact for Dental Procurement:
Shanghai Carejoy Medical Co., LTD
Factory: No. 1888, Hengfeng Road, Baoshan District, Shanghai, China
Email: [email protected]
WhatsApp: +86 159 5127 6160 (24/7 English-speaking support)
Verification: Request factory audit report via QR code on official website: www.carejoydental.com
Implementation Roadmap
- Q1 2026: Shortlist 3 suppliers with valid EU MDR certificates. Conduct virtual factory tour.
- Q2 2026: Order pre-shipment inspection (PSI) of 2 demo units. Validate dose accuracy per IEC 61223-3-5.
- Q3 2026: Negotiate DDP terms with Carejoy or equivalent tier-1 supplier. Lock in 2026 pricing before Q4 raw material hikes.
Final Advisory: Avoid suppliers without physical factory addresses in Shanghai/Suzhou industrial zones. 2026 enforcement actions by CNCA have eliminated 127 uncertified dental X-ray manufacturers. Partner exclusively with suppliers demonstrating full regulatory chain-of-custody documentation. Shanghai Carejoy’s 19-year export history (2005-2026) provides critical supply chain resilience amid ongoing US-China tariff fluctuations.
Frequently Asked Questions
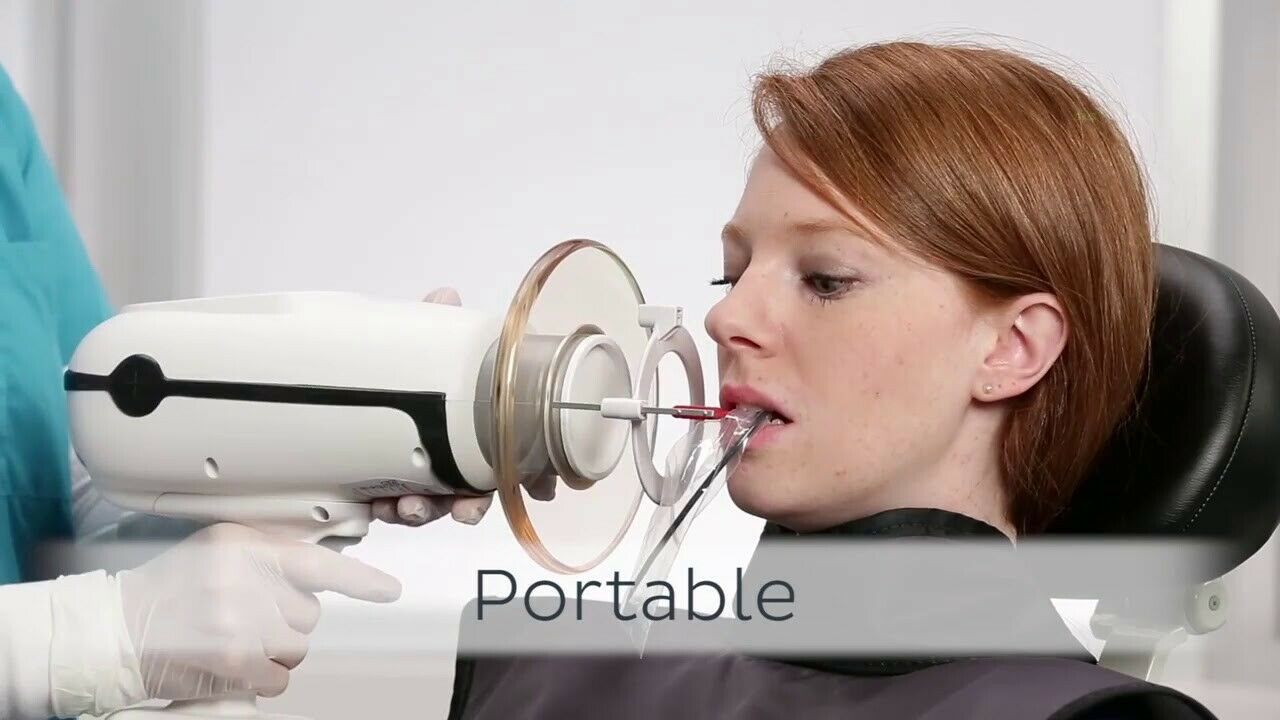
Professional Dental Equipment Guide 2026
Frequently Asked Questions: Nomad™ Pro 2 Portable X-Ray System
Designed for dental clinics and equipment distributors evaluating next-generation intraoral imaging solutions.
| Question | Answer |
|---|---|
| 1. What is the operating voltage range for the Nomad Pro 2 Portable X-Ray in 2026, and is it compatible with global power standards? | The Nomad Pro 2 operates on a rechargeable lithium-ion battery system, eliminating the need for direct mains voltage during use. The integrated charger supports 100–240 V AC, 50/60 Hz, making it fully compatible with international power standards. This universal input ensures seamless deployment across North America, Europe, Asia, and other global markets without voltage converters. Clinics and distributors should verify local regulatory compliance (e.g., CE, FDA, Health Canada) for commercial distribution. |
| 2. Are spare parts for the Nomad portable X-ray readily available, and what is the recommended inventory for clinics and distributors? | Yes, all critical spare components—including collimator tips, position-indicating devices (PIDs), battery packs, charging docks, and protective lead shields—are available through authorized distributors and the manufacturer’s global logistics network. For 2026, we recommend clinics maintain at least one spare battery and PID, while distributors should stock core consumables and high-wear items (e.g., trigger assemblies, O-rings, and alignment caps) to support regional service demands. Firmware and hardware revisions are tracked via serial number to ensure part compatibility. |
| 3. Does the Nomad Pro 2 require professional installation, and what are the setup requirements in a clinical environment? | No professional installation is required. The Nomad Pro 2 is designed for plug-and-play operation: simply charge the unit, attach the PID, and follow the on-screen startup protocol. Setup requires only a standard power outlet for charging and secure storage when not in use. No shielding modifications, dedicated circuits, or room certifications are needed, making it ideal for mobile practices, multi-operator environments, and clinics with space constraints. Training modules and digital onboarding are provided via the Aribex Connect platform. |
| 4. What warranty coverage is provided with the Nomad Pro 2, and are extended service plans available? | The Nomad Pro 2 comes with a comprehensive 3-year limited warranty covering defects in materials and workmanship, including the X-ray tube head and internal electronics. Battery packs are covered for 1 year with performance retention above 80% of original capacity. Extended warranty options are available for up to 5 years, including accidental damage protection and priority technical support. Distributors may offer bundled service agreements tailored to regional clinical needs and high-volume usage scenarios. |
| 5. How are firmware updates and technical support handled under warranty, and is remote diagnostics supported? | Firmware updates are delivered securely via the Aribex Connect mobile app or web portal, ensuring regulatory-compliant performance enhancements and new feature rollouts. Remote diagnostics are supported through Bluetooth-enabled troubleshooting, allowing authorized technicians to assess unit performance without physical intervention. Technical support is available 24/7 to clinics and distributors through dedicated regional service centers, with SLAs for response and resolution included in extended warranty packages. |
Need a Quote for Nomad Portable X Ray Machine?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


