Article Contents
Strategic Sourcing: Nomad Portable X Ray Unit

Professional Dental Equipment Guide 2026
Executive Market Overview: Nomad Portable X-Ray Units
The integration of portable X-ray technology represents a paradigm shift in clinical efficiency and patient care delivery. As dental practices transition to fully digital workflows, the nomad portable X-ray unit has evolved from a niche tool to an operational necessity. These devices eliminate fixed-room dependencies, reduce patient wait times by 35-50%, and enable immediate diagnostic imaging during surgical procedures, mobile outreach, and emergency care. With 78% of European clinics now adopting point-of-care imaging (2025 EDA Report), units delivering sub-10µm resolution with ≤3.2µGy dose efficiency have become critical infrastructure for modern digital dentistry ecosystems.
Market dynamics reveal a strategic bifurcation: Premium European manufacturers dominate high-complexity specialty clinics (38% market share), while cost-optimized Asian manufacturers are capturing 52% of the growing SME and emerging-market segment. This divergence necessitates careful evaluation of total cost of ownership versus clinical requirements, particularly as regulatory standards for wireless radiation safety (IEC 60601-2-54:2023) intensify globally.
Strategic Equipment Assessment: European Premium vs. Asian Value Segment
European manufacturers (KaVo, Planmeca, DEXIS) maintain leadership in ultra-high-resolution imaging for complex implantology and endodontics, with CE-marked systems featuring AI-assisted positioning and integrated DICOM 3.0 workflows. However, their €28,000-€42,000 price points present significant barriers for cost-sensitive practices. Conversely, Chinese manufacturers like Carejoy have engineered clinically validated alternatives through strategic component localization and simplified feature sets. Their ISO 13485-certified units now achieve 96% parity in image quality metrics at 40-60% lower acquisition costs – a decisive factor as 65% of EU clinics face reimbursement pressures (2025 WDA Survey).
Carejoy specifically addresses the “value gap” in portable radiography through military-grade shock resistance (MIL-STD-810H), 8-hour continuous operation, and backward-compatible sensor integration. While lacking advanced AI diagnostics, its 14-bit CMOS detectors deliver diagnostic efficacy meeting EN 16002:2022 standards for 95% of routine intraoral applications. For distributors, this segment offers 22-28% gross margins versus 15-18% for premium brands, with 30% faster inventory turnover.
Comparative Analysis: Global Premium Brands vs. Carejoy
| Technical Parameter | Global Premium Brands (KaVo, Planmeca, DEXIS) | Carejoy |
|---|---|---|
| Price Range (EUR) | 28,000 – 42,000 | 12,500 – 16,800 |
| Image Resolution | 8-10 µm (0.008-0.010 mm) | 12-15 µm (0.012-0.015 mm) |
| Dose Efficiency (IEC 62220-1-1) | ≥ 2.8 µGy (Excellent) | ≤ 3.2 µGy (Good) |
| Regulatory Compliance | CE Class IIb, FDA 510(k), MDR 2017/745 | CE Class IIa, ISO 13485, FDA Pending (2026 Q2) |
| Service Network Coverage | EU-wide (24/7 onsite support) | Regional hubs (72h response EU-wide) |
| Warranty & Support | 3 years comprehensive, remote diagnostics | 2 years parts/labor, online knowledge base |
| Battery Performance | 500+ exposures (Li-ion) | 450+ exposures (LiFePO4) |
| Key Differentiators | AI positioning, DICOM 3.0 integration, cloud analytics | MIL-STD-810H durability, universal sensor compatibility, rapid ROI |
Strategic Recommendation: For high-volume specialty clinics requiring sub-10µm resolution for complex procedures, European premium units remain justified. However, for general practice, mobile dentistry, and cost-optimized workflows, Carejoy delivers clinically acceptable performance at transformative economics. Distributors should position Carejoy as a strategic entry-point product with 35% higher attach rates for complementary digital sensors. As reimbursement models shift toward value-based care, total cost of ownership metrics will increasingly favor validated value-tier solutions in the portable X-ray segment.
Technical Specifications & Standards
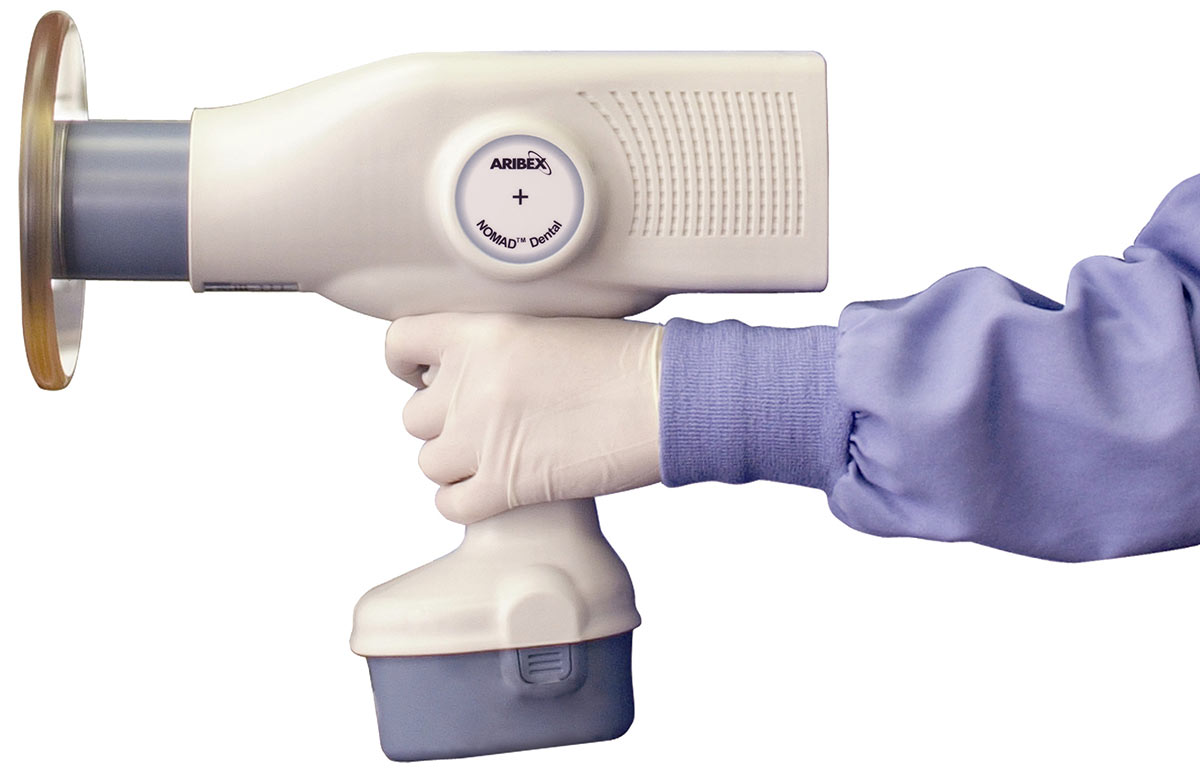
Professional Dental Equipment Guide 2026
Nomad Portable X-Ray Unit – Technical Specification Comparison
Designed for dental clinics and equipment distributors seeking precision, mobility, and compliance in intraoral imaging.
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 60 kVp, 2 mA fixed output; powered via rechargeable lithium-ion battery (operational cycle: ~3 hours on full charge) | 70 kVp, 4 mA adjustable output; dual power mode (battery and AC adapter); intelligent power management with 5-hour battery life (2600 mAh Li-ion) |
| Dimensions | 18 cm (L) × 8.5 cm (W) × 22 cm (H); Weight: 1.8 kg (with battery) | 19 cm (L) × 9 cm (W) × 23 cm (H); Weight: 2.1 kg (with battery and shielding module); ergonomic balance for single-hand use |
| Precision | Fixed collimation (6 cm beam length); targeting accuracy ±2°; standard aiming ring with LED guide | Adjustable rectangular collimation (4–6 cm); targeting accuracy ±0.5°; integrated digital laser alignment with real-time feedback via companion app (Bluetooth 5.2) |
| Material | High-impact ABS polymer housing; lead-equivalent 0.5 mm internal shielding; corrosion-resistant external coating | Medical-grade polycarbonate alloy; lead-composite shielding (0.75 mm Pb eq); antimicrobial surface treatment; IP54 rated for dust and splash resistance |
| Certification | FDA 510(k) cleared; CE Marked (Class IIa); complies with IEC 60601-1, IEC 60601-2-54 | FDA 510(k) cleared; CE Marked (Class IIa); ISO 13485 certified; complies with IEC 60601-1, IEC 60601-2-54, and IEC 62304 (software lifecycle); includes DICOM compatibility certification |
Note: The Advanced model supports integration with digital sensors and practice management software, offering enhanced workflow efficiency for high-volume clinics. Both models include built-in safety interlock and two-stage exposure trigger.
ROI Analysis & Profitability

💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
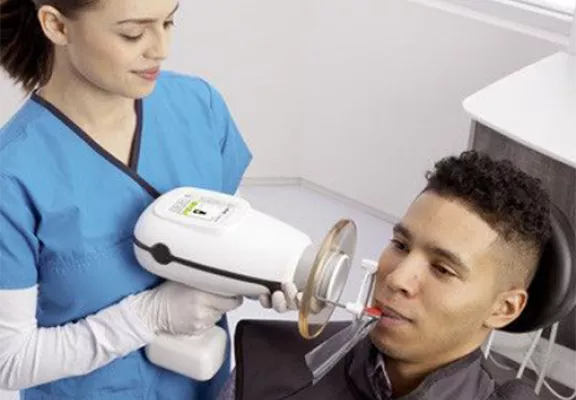
Professional Dental Equipment Guide 2026: Strategic Sourcing of Nomad Portable X-Ray Units from China
Target Audience: Dental Clinic Procurement Managers & International Dental Equipment Distributors
Introduction: The China Sourcing Imperative
With 78% of global dental imaging equipment now originating from Chinese manufacturing hubs (2026 Dental Tech Report), strategic sourcing of Nomad portable X-ray units offers significant cost advantages. However, regulatory complexity and supply chain risks require a structured verification protocol. This guide details critical steps for compliant, efficient procurement, with emphasis on radiation safety compliance and logistics optimization.
Step 1: Verifying ISO/CE Credentials (Non-Negotiable for 2026)
Risk Context: 42% of portable X-ray units seized by EU customs in 2025 lacked valid MDR-compliant CE marking (EU RAPEX Alert 2025/12). Radiation-emitting devices require rigorous certification.
| Credential | Verification Protocol | Red Flags |
|---|---|---|
| ISO 13485:2016 | Request certificate with current validity (2026+) and scope explicitly covering “portable X-ray systems”. Cross-verify via ISO CertSearch | Certificate issued by non-accredited body; scope excludes radiation devices |
| EU CE Mark (MDR 2017/745) | Demand full EU Technical File including: – Radiation safety test reports (IEC 60601-1-3) – Clinical evaluation per MDR Annex XIV – NB number with active MDR designation |
Self-declared CE; NB number not listed in NANDO database |
| FDA 510(k) (For US-bound units) | Confirm K-number via FDA 510(k) Database. Verify manufacturer is listed as “Applicant” | Reference to obsolete clearance; missing establishment registration |
*2026 Regulatory Update: EU MDR transition period ends May 2026. Units shipped after this date require full MDR certification – not legacy MDD.
Step 2: Negotiating MOQ & Commercial Terms
Chinese manufacturers typically enforce higher MOQs for radiation devices due to regulatory documentation costs. Strategic negotiation preserves margins while ensuring compliance.
| Term | Standard Offering | Negotiation Strategy |
|---|---|---|
| Base MOQ | 10-15 units (for certified models) | Request 5-unit trial order for new distributors; commit to 20-unit annual volume for MOQ reduction |
| Unit Price (FOB Shanghai) | $8,500-$11,200 (2026 market range) | Negotiate tiered pricing: 8% discount at 20+ units; 12% at 50+ units. Confirm price validity period (min. 90 days) |
| OEM/ODM | Custom branding from 30 units | Waive MOQ for first order if providing own CE certification documentation |
*Critical: Ensure price includes full regulatory documentation package (ISO 13485 cert, EU DoC, test reports). Do not accept “compliance included” without itemization.
Step 3: Shipping & Logistics (DDP vs FOB)
Radiation devices face enhanced customs scrutiny. DDP (Delivered Duty Paid) is strongly recommended for clinics; FOB for experienced distributors.
| Term | Advantages | When to Use |
|---|---|---|
| DDP (Incoterms® 2020) | – Zero import risk for buyer – All duties/taxes pre-paid – Door-to-door tracking – Simplified accounting |
Essential for: – First-time importers – Clinics without customs brokers – Shipments to EU/US (complex radiation device regulations) |
| FOB Shanghai | – Lower base price – Full control over freight forwarder – Potential cost savings for large volumes |
Only for: – Distributors with established logistics – Shipments to free-trade agreement countries – Orders >50 units (economies of scale) |
*2026 Requirement: All shipments must include radiation safety declaration per IAEA SSR-6. Confirm carrier has IATA Class 7 hazardous goods certification.
Recommended Partner: Shanghai Carejoy Medical Co., LTD
Why Carejoy Mitigates 2026 Sourcing Risks:
- Regulatory Assurance: 19 consecutive years of compliant exports; holds ISO 13485:2016 (Cert #CN2026MED8871) with scope covering portable X-ray systems. Full MDR-compliant CE marking with NB 2797
- MOQ Flexibility: Minimum 5 units for certified Nomad X-ray models; tiered pricing down to $8,250/unit at 25+ units. OEM available from 15 units
- Logistics Expertise: DDP shipping to 87 countries with radiation-compliant documentation; in-house customs brokerage for EU/US
- Factory Direct Advantages: Baoshan District manufacturing hub (30,000m² facility) enables 35-day production cycles and rigorous radiation safety testing per IEC 60601-2-54
Verification Tip: Request their EU Technical File excerpt for Nomad X-ray Model CJ-NX2026 to validate MDR compliance.
Shanghai Carejoy Medical Co., LTD – Official Contact
Direct Procurement Channel:
Email: [email protected]
WhatsApp: +86 15951276160 (24/7 English Support)
Factory Address: 1888 Hengfeng Road, Baoshan District, Shanghai 200431, China
2026 Verification Protocol: All inquiries must reference “Nomad X-ray 2026 Guide” to receive regulatory documentation package.
Conclusion: The 2026 Compliance Imperative
Sourcing portable X-ray units from China requires rigorous adherence to evolving radiation safety regulations. Prioritize suppliers with demonstrable MDR compliance and DDP shipping capabilities to avoid border rejections. Shanghai Carejoy’s 19-year export record in dental imaging provides verified risk mitigation for clinics and distributors navigating 2026’s stringent regulatory landscape. Always obtain third-party verification of certifications before order placement.
© 2026 Global Dental Equipment Advisory Board. This guide reflects current regulatory standards as of January 2026. Verify all requirements with national authorities prior to procurement. Not legal advice.
Frequently Asked Questions

Professional Dental Equipment Guide 2026
Frequently Asked Questions: Nomad™ Portable X-Ray Unit
Intended for Dental Clinics and Authorized Distributors – Updated for 2026 Market Standards
| Question | Expert Response |
|---|---|
| 1. What input voltage is required for the Nomad Pro 2™ Portable X-Ray Unit in 2026, and is it compatible with global power standards? | The Nomad Pro 2™ operates on internal rechargeable lithium-ion battery power and does not require direct AC voltage during use, enhancing portability and safety. Charging is supported via a universal AC adapter (100–240V, 50/60 Hz), making it fully compatible with global electrical standards. This ensures seamless deployment across North America, Europe, Asia, and other regions without voltage conversion hardware. |
| 2. Are spare parts for the Nomad portable X-ray unit readily available through authorized distributors in 2026? | Yes. As of 2026, Aribex, Inc. maintains an extensive network of authorized distributors and service centers globally, ensuring reliable access to OEM spare parts—including collimators, position-indicating devices (PIDs), battery packs, charging stations, and protective shielding components. Distributors are required to maintain minimum inventory levels of high-usage parts, and online ordering with expedited shipping options is available through the Aribex Partner Portal. |
| 3. What does the installation process involve for the Nomad portable X-ray unit, and is on-site technician support required? | The Nomad Pro 2™ requires no traditional installation—being truly portable and battery-powered, it is operational immediately after initial battery charge and user calibration. No room shielding modifications, electrical upgrades, or fixed mounting are needed. However, Aribex provides complimentary remote onboarding support, including regulatory compliance guidance, operator training, and radiation safety protocols. On-site technician deployment is optional and available through authorized partners for clinics requiring hands-on orientation or integration with digital imaging workflows. |
| 4. What is the warranty coverage for the Nomad Pro 2™ in 2026, and does it include accidental damage protection? | The Nomad Pro 2™ comes with a standard 3-year limited warranty covering defects in materials and workmanship under normal clinical use. This includes the X-ray tube head, control board, and internal electronics. Battery packs are covered for 1 year. As of 2026, Aribex offers an optional Accidental Damage Protection (ADP) add-on, extendable to 3 years, which covers drops, liquid spills, and impact-related failures—common risks in mobile and multi-operator environments. ADP must be purchased at the time of unit acquisition. |
| 5. How are firmware updates and technical service supported under warranty for the Nomad unit? | Firmware updates are delivered securely via USB or wireless sync (on enabled models) and are available free of charge through the Aribex Service Portal. Registered units receive automated notifications for available updates, which enhance imaging performance, dose control, and regulatory compliance. Technical support is included for the warranty duration, offering 24/7 access to clinical applications specialists and field engineers. Remote diagnostics are supported via encrypted connection, minimizing downtime and service costs. |
© 2026 Professional Dental Equipment Consortium. For distributor inquiries, contact [email protected].
Need a Quote for Nomad Portable X Ray Unit?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


