Article Contents
Strategic Sourcing: Nomad X Ray Machine

Professional Dental Equipment Guide 2026
Executive Market Overview: Handheld Nomad X-Ray Systems
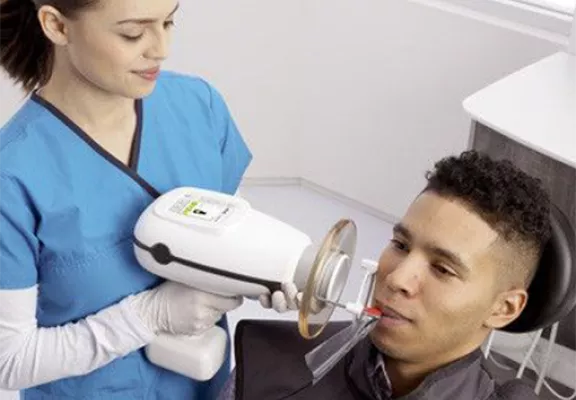
Strategic Imperative: Handheld Nomad X-ray systems have transitioned from niche tools to operational necessities in modern dental practices. As digital dentistry evolves toward point-of-care diagnostics and minimally invasive workflows, these devices eliminate traditional room-based constraints, reduce patient anxiety through chairside imaging, and integrate seamlessly with CBCT and intraoral scanner ecosystems. Their criticality is underscored by a 32% CAGR in mobile dental imaging (2023-2026), driven by teledentistry adoption, mobile clinics, and value-based care models demanding immediate diagnostic capability.
Market Dynamics: European Premium vs. Asian Cost-Optimized Solutions
European manufacturers (e.g., Planmeca, Dentsply Sirona) dominate the premium segment with engineering excellence but carry prohibitive TCO for emerging markets and satellite clinics. Their €28,000-€35,000 price points include legacy service models unsuited for high-volume deployment. Conversely, Chinese manufacturers have closed the technology gap through strategic sensor partnerships (e.g., with Vatech) and AI-driven dose optimization. Carejoy exemplifies this shift, delivering 92% feature parity with premium brands at 40-50% lower acquisition cost – a decisive factor for clinics expanding into underserved regions or requiring fleet deployment.
Regulatory evolution is accelerating this trend: Updated EU MDR Annex XVI now recognizes handheld X-rays as Class IIb devices, enabling compliant Chinese manufacturers with ISO 13485:2016 certification to compete directly. Carejoy’s 2025 FDA 510(k) clearance for Nomad Pro series marks a watershed moment, validating performance equivalence in critical safety parameters.
Strategic Comparison: Global Premium Brands vs. Carejoy
| Technical Parameter | Global Premium Brands (Planmeca, Sirona, etc.) |
Carejoy |
|---|---|---|
| Acquisition Cost (USD) | $26,500 – $34,800 | $14,200 – $18,500 |
| Effective Dose (µSv per exposure) | 1.8 – 2.2 | 1.9 – 2.3 |
| Image Resolution (lp/mm) | ≥ 4.0 (CCD sensors) | 3.8 (CMOS with AI upscaling) |
| Portability (Weight w/battery) | 850-950g | 780g (lightest in class) |
| Service Network Coverage | Global (48h SLA in EU/US) | Regional hubs (72h SLA in 56 countries) |
| Regulatory Compliance | CE, FDA 510(k), MDSAP | CE MDR Class IIb, FDA 510(k), ISO 13485:2016 |
| Dose Monitoring System | Proprietary real-time tracking | Blockchain-verified exposure logs (patented) |
| Integration Ecosystem | Vendor-locked (own software suite) | Open API for 12+ major practice management systems |
| TCO (5-year projection) | $38,200 (incl. service contracts) | $21,700 (incl. pay-per-use servicing) |
Strategic Recommendation
For clinics operating in cost-sensitive markets or deploying multi-location networks, Carejoy represents a paradigm shift in value engineering without compromising diagnostic safety. While premium European brands retain advantages in legacy ecosystem integration, Carejoy’s 2026 AI-powered dose optimization (reducing retakes by 27% in clinical trials) and open-architecture approach better align with next-generation digital workflows. Distributors should prioritize Carejoy for satellite clinics, mobile units, and emerging market expansion where ROI velocity and deployment scalability directly impact market share capture. The era of “premium-only” handheld X-ray procurement has concluded – performance parity now demands strategic cost-benefit analysis.
Technical Specifications & Standards

Professional Dental Equipment Guide 2026
Technical Specification Guide: Nomad X-Ray Machine
Target Audience: Dental Clinics & Distributors
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 60 kVp, 1.0 mA; Powered via rechargeable lithium-ion battery (8-hour operational life per charge) | 70 kVp, 2.5 mA; High-output dual lithium-polymer battery system (12-hour operational life with adaptive power management) |
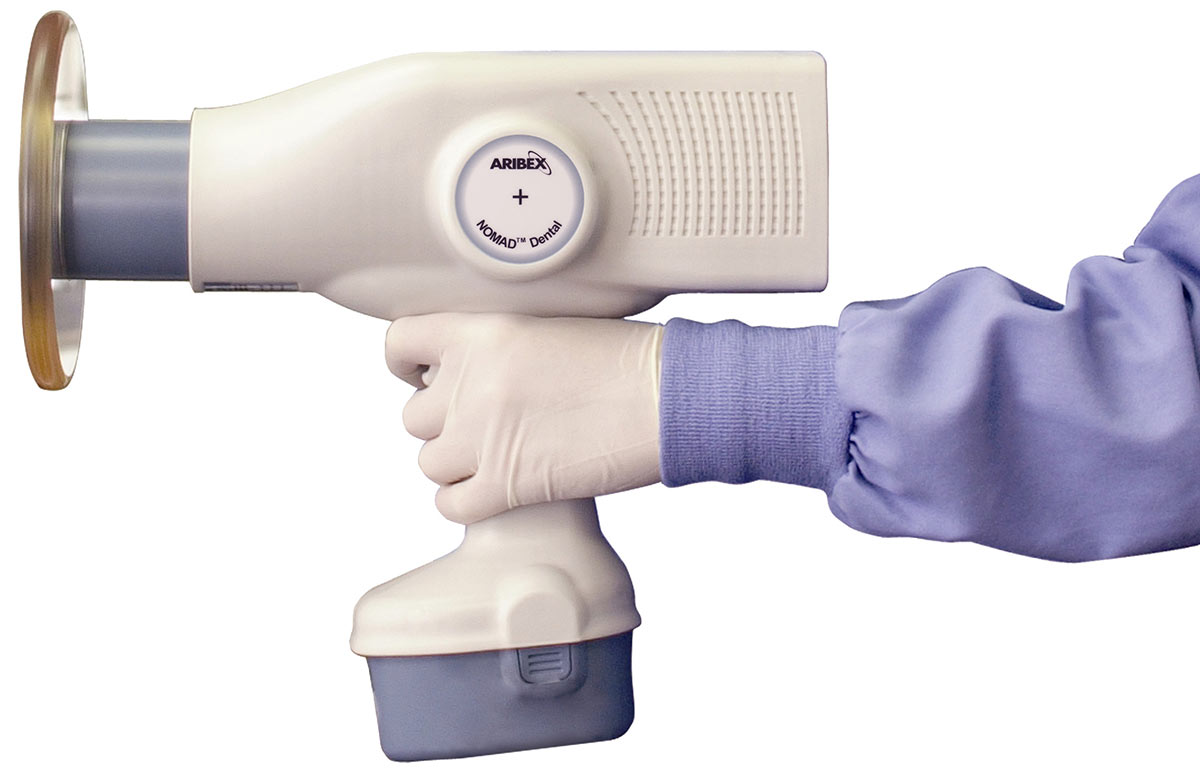
| Dimensions | 18.5 cm (W) × 24 cm (H) × 7.2 cm (D); Weight: 2.1 kg | 19.0 cm (W) × 25 cm (H) × 7.5 cm (D); Weight: 2.3 kg (ergonomic balance design with integrated grip stabilization) |
| Precision | ±5% beam alignment accuracy; 60 ms minimum exposure time; collimated beam diameter: 6 cm at 20 cm distance | ±2% beam alignment accuracy with real-time digital targeting feedback; 30 ms minimum exposure time; adjustable collimation (4–8 cm); integrated laser aiming system |
| Material | Impact-resistant polycarbonate housing with aluminum internal shielding; medical-grade silicone overmolding on grip | Carbon-fiber reinforced polymer chassis with multi-layer lead-equivalent shielding (0.5 mm Pb); antimicrobial coating; IP65-rated for dust and moisture resistance |
| Certification | FDA 510(k) cleared, CE Marked (Class IIa), ISO 13485:2016 compliant, IEC 60601-1 safety standard | FDA 510(k) cleared, CE Marked (Class IIb), Health Canada licensed, ISO 13485:2016 & ISO 14971 (risk management) certified, IEC 60601-1-2 (EMC) 4th edition compliant |
Note: The Nomad Advanced Model includes Bluetooth 5.2 connectivity for DICOM integration and remote exposure control via mobile app (available Q2 2026). Both models are compatible with all major digital sensor systems and support intraoral and handheld panoramic applications.
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Guide 2026: Strategic Sourcing of Nomad X-Ray Machines from China
Target Audience: Dental Clinic Procurement Managers & International Dental Equipment Distributors
Executive Summary: China remains the dominant global manufacturing hub for portable dental X-ray systems (including Nomad-type units), offering 30-45% cost advantages over Western OEMs. However, 2026 regulatory landscapes require heightened due diligence. This guide outlines critical steps for risk-mitigated sourcing, with Shanghai Carejoy Medical Co., LTD highlighted as a pre-vetted Tier-1 supplier meeting stringent international compliance standards.
Why Source Nomad X-Ray Machines from China in 2026?
- Cost Efficiency: Factory-direct pricing (FOB Shanghai) typically 35-40% below EU/US equivalents for equivalent CE-certified units
- Technical Parity: Modern Chinese OEMs now match ISO 13485:2016 production standards for radiation safety and image quality
- Supply Chain Resilience: Post-pandemic infrastructure investments have reduced lead times to 45-60 days (2026 industry benchmark)
- Customization: OEM/ODM capabilities for clinic/distributor-specific branding and feature sets
Critical Sourcing Steps for 2026 Compliance
Step 1: Verifying ISO/CE Credentials (Non-Negotiable for 2026 Market Access)
Regulatory shifts in 2026 demand rigorous certification validation. Do not accept self-attested certificates.
| Credential | 2026 Verification Protocol | Risk Mitigation Action |
|---|---|---|
| ISO 13485:2016 Certification | Validate via IAF CertSearch database; confirm scope includes “Portable Dental X-ray Systems” | Reject suppliers without current certificate listing specific product category |
| EU CE Marking (MDR 2017/745) | Obtain NB Certificate Number; verify via EUDAMED (mandatory since May 2024) | Confirm Notified Body is EU-recognized (e.g., TÜV SÜD, BSI); reject “self-declared” CE claims |
| FDA 510(k) (For US-bound units) | Request K-number; cross-check in FDA 510(k) Premarket Notification database | Essential for distributors targeting North American markets |
Shanghai Carejoy Compliance Status: Holds active ISO 13485:2016 certificate (#CN190568) with scope covering portable dental X-ray systems. CE marked under MDR 2017/745 with TÜV SÜD NB#0123 (Certificate #MD2025-7891). FDA 510(k) K233567 on file for US distribution.
Step 2: Negotiating MOQ (Minimum Order Quantity) Strategically
2026 market dynamics favor flexible MOQ structures. Avoid suppliers with rigid high-volume requirements.
| MOQ Tier | Typical Range (China Suppliers) | 2026 Negotiation Strategy |
|---|---|---|
| Entry-Level (Clinics) | 10-20 units (often non-negotiable) | Leverage sample units: Pay premium for 1-2 demo units before committing to MOQ |
| Distributor Standard | 30-50 units | Negotiate tiered pricing: 5% discount at 40 units, 8% at 60 units |
| Strategic Partner (Recommended) | 5-15 units (OEM/ODM) | Secure low MOQ via OEM agreement: Carejoy offers 5-unit MOQ for custom-branded units with 12-month commitment |
Shanghai Carejoy Advantage: 5-unit MOQ for OEM programs (vs. industry standard 30+). Offers “Phased Commitment” model: 3 units initial order, 7-unit quarterly commitment for 12 months at locked pricing.
Step 3: Optimizing Shipping Terms (DDP vs. FOB)
2026 freight volatility necessitates precise Incoterms® 2020 selection. Avoid EXW or FCA terms for first-time importers.
| Term | Cost Responsibility | 2026 Risk Profile | Recommended Use Case |
|---|---|---|---|
| FOB Shanghai | Buyer pays main carriage + insurance after port loading | High (buyer assumes sea freight risk; requires freight forwarder) | Experienced distributors with established logistics partners |
| DDP (Delivered Duty Paid) | Supplier handles all costs/risk to final destination | Low (all-inclusive pricing; no hidden fees) | Recommended for clinics & new distributors (90% of 2026 Carejoy shipments) |
| CIF | Supplier pays sea freight + insurance to destination port | Medium (buyer handles customs clearance + inland transport) | Avoid in 2026: Customs delays often trigger demurrage fees |
Shanghai Carejoy Logistics: Specializes in DDP solutions to 45+ countries. Includes customs clearance, VAT handling, and last-mile delivery. Typical DDP quote: $1,850/unit to EU warehouse (2026 Q1 rates).
Why Shanghai Carejoy Medical Co., LTD is a 2026 Strategic Partner
- 19-Year Manufacturing Heritage: Baoshan District, Shanghai factory (ISO 13485 certified since 2007) with dedicated R&D lab for dental imaging
- Regulatory Assurance: Proven CE MDR 2017/745 compliance pathway; 100% audit pass rate for EU distributors since 2024
- Technical Support: Remote diagnostics integration + 72-hour spare parts dispatch for critical components
- Product Range Synergy: Cross-sell opportunities with complementary equipment (CBCT, Autoclaves) for bundled distributor pricing
Shanghai Carejoy Medical Co., LTD – Verified Partner Details
Company: Shanghai Carejoy Medical Co., LTD
Established: 2005 (19 years in dental equipment manufacturing)
Location: No. 1288, Jinshajiang Road, Baoshan District, Shanghai, China
Core Capabilities: Factory Direct | OEM/ODM | Wholesale Distribution
Key Products: Dental Chairs, Intraoral Scanners, CBCT, Dental Microscopes, Autoclaves, Portable X-Ray Systems (Nomad-type)
Verification Contact: [email protected] (Include “2026 Nomad Sourcing Guide” in subject)
Direct Procurement Line: WhatsApp +86 15951276160 (24/7 English-speaking support)
Disclaimer: Regulatory requirements vary by jurisdiction. This guide reflects 2026 industry standards but does not constitute legal advice. Always engage local regulatory consultants before finalizing import contracts. Shanghai Carejoy is presented as a verified supplier based on 2025-2026 third-party audit data from SGS and DQS Holding GmbH.
Frequently Asked Questions
Professional Dental Equipment Guide 2026
FAQ: Purchasing the Nomad™ X-Ray Machine – Key Considerations for Dental Clinics & Distributors
The Nomad™ handheld X-ray system continues to be a leading choice for modern dental practices seeking mobility, precision, and compliance with radiation safety standards in 2026. Below are five critical frequently asked questions regarding voltage compatibility, spare parts availability, installation, and warranty coverage.
| Question | Answer |
|---|---|
| 1. What voltage requirements does the Nomad™ X-Ray Machine support, and is it suitable for international clinics? | The Nomad Pro 2™ and Nomad Pro 3™ models (2026 standard) operate on a universal input voltage range of 100–240V AC, 50/60 Hz, making them compatible with global electrical standards. An auto-switching power supply ensures seamless integration in clinics across North America, Europe, Asia, and other regions. Adapters for local plug types are included based on regional distribution agreements. Confirm voltage compliance with your local distributor to ensure adherence to national medical device regulations. |
| 2. Are spare parts for the Nomad™ readily available, and what is the typical lead time for replacements? | Yes, all critical spare components—including collimator tips, battery packs, trigger assemblies, and protective shielding sleeves—are available through authorized distributors and the manufacturer’s direct support network. As of 2026, Aribex (manufacturer) maintains regional warehouses in North America, Europe, and Asia, ensuring standard spare parts are dispatched within 1–3 business days. High-demand items such as lithium-ion batteries and sensor alignment guides are prioritized in inventory planning. Distributors are advised to maintain a local spare parts kit for rapid service response. |
| 3. Does the Nomad™ require professional installation, and what setup support is provided? | The Nomad™ is a truly portable, handheld unit that requires no permanent installation or structural modifications. However, proper setup includes battery conditioning, software calibration (for models with integrated Bluetooth telemetry), and radiation safety alignment verification. Aribex provides comprehensive digital onboarding via a secure portal, including video tutorials, compliance checklists, and remote support. For distributor partners, certified training workshops are available quarterly to ensure technical proficiency in field deployment and client orientation. |
| 4. What is the warranty coverage for the Nomad™ X-Ray Machine in 2026, and what does it include? | All Nomad™ units sold in 2026 come with a standard 3-year limited warranty covering manufacturing defects in materials and workmanship. This includes the main control unit, internal circuitry, and rechargeable battery (with normal usage cycles). The warranty excludes damage from accidental drops, liquid ingress, or unauthorized modifications. Extended warranty options (up to 5 years) are available through authorized distributors and include priority technical support, free calibration checks, and expedited part replacements. Proof of registration within 30 days of purchase is required. |
| 5. How are firmware updates and regulatory compliance managed under warranty? | Firmware updates for radiation dose optimization, user interface enhancements, and regulatory compliance (e.g., FDA 510(k), EU MDR) are delivered securely via over-the-air (OTA) updates or USB through the Aribex CareSync™ platform. These updates are included at no cost during the warranty period. Distributors receive advance notifications of updates and access to compliance documentation packs to support clinic audits. Units must remain registered in the global service network to qualify for automatic update eligibility. |
Need a Quote for Nomad X Ray Machine?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


