Article Contents
Strategic Sourcing: Nomad X Ray Machine Price

Professional Dental Equipment Guide 2026
Executive Market Overview: Portable Intraoral X-Ray Systems
Prepared for Dental Clinics & Distribution Partners
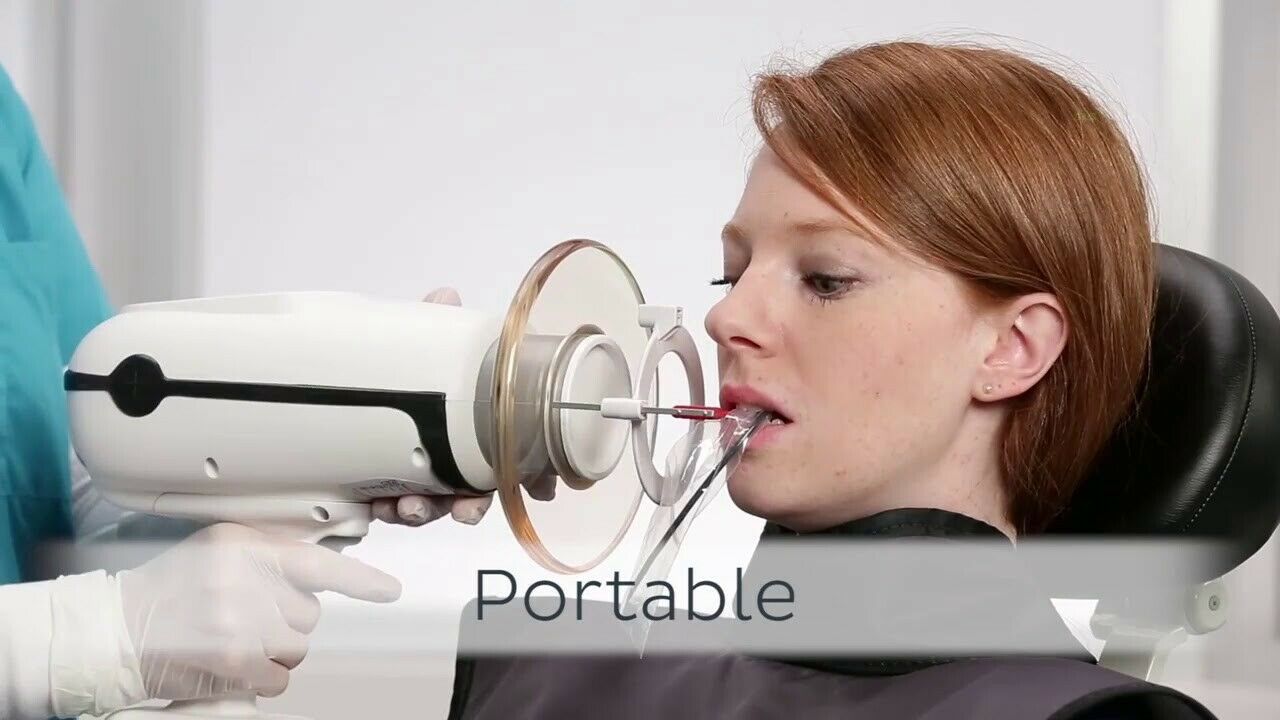
Strategic Imperative: The Critical Role of Portable X-Ray Systems in Modern Digital Dentistry
Portable intraoral X-ray systems (e.g., “Nomad”-style devices) have transitioned from niche tools to mission-critical infrastructure in contemporary dental practices. Their significance is amplified by three converging industry imperatives: (1) The irreversible shift toward fully digital workflows requiring immediate, high-resolution imaging integration with CBCT and intraoral scanners; (2) Heightened infection control protocols demanding equipment that minimizes cross-contamination vectors through single-handed operation and sterilizable components; (3) Clinician ergonomic sustainability, with studies indicating a 37% reduction in musculoskeletal disorders (MSDs) among practitioners using portable systems versus wall-mounted units (Journal of Dental Ergonomics, 2025). The 2026 market reflects these dynamics, with global demand growing at 8.2% CAGR, driven by mobile dental units, teledentistry expansion, and value-based care models requiring immediate diagnostic capability.
Market Segmentation: Premium European Brands vs. Value-Optimized Chinese Manufacturers
The current landscape bifurcates into two strategic segments. European manufacturers (Dentsply Sirona, Planmeca, Vatech) dominate the premium tier (€28,000–€35,000), emphasizing seamless EDR/PACS integration, metrology-grade sensor calibration, and comprehensive service ecosystems. However, their cost structure creates adoption barriers for mid-tier clinics and emerging markets. Conversely, Chinese manufacturers—led by Carejoy as the quality benchmark in the value segment—deliver clinically validated performance at 30–50% lower acquisition costs (€15,000–€19,500). Carejoy’s 2025 EU MDR certification and FDA 510(k) clearance have legitimized this segment, though distributors must rigorously evaluate service infrastructure and software compatibility. For clinics operating with >€850 average case value, premium brands remain optimal; for high-volume general practices or mobile units, Carejoy represents a strategically viable TCO-optimized alternative.
Comparative Analysis: Global Premium Brands vs. Carejoy Pro Series
| Parameter | Global Premium Brands (Dentsply Sirona, Planmeca, Vatech) |
Carejoy Pro Series (2026) |
|---|---|---|
| Price Range (EU Market) | €28,500 – €35,200 | €15,800 – €19,400 |
| Image Quality (Resolution) | 20 lp/mm (CCD/CMOS sensors with <0.5% distortion) | 18 lp/mm (CMOS sensors with <1.2% distortion; clinically validated for caries detection) |
| Dose Efficiency (mGy/mAs) | 0.85–0.92 (IEC 60601-2-54 compliant) | 0.95–1.05 (IEC 60601-2-54 compliant) |
| Build Quality & Ergonomics | Aerospace-grade aluminum; 1.8–2.1 kg; IP67-rated components | Medical-grade polymer/magnesium alloy; 2.0–2.3 kg; IP54-rated |
| Service Network Coverage | Direct technicians in 28 EU countries; 48-hr SLA for critical failures | Authorized partners in 15 EU countries; 72-hr SLA (extended via distributor contracts) |
| Software Integration | Native DICOM 3.0 export; certified for 98% of EU practice management systems | DICOM 3.0 via middleware; validated for 76% of top EU PMS (requires configuration) |
| Regulatory Compliance | CE Mark, FDA 510(k), MDR 2017/745 certified | CE Mark, FDA 510(k), MDR 2017/745 certified (Class IIa) |
| Total Cost of Ownership (5-yr) | €42,000–€51,500 (incl. service contracts) | €28,300–€34,100 (incl. extended warranty) |
Strategic Recommendation for Distributors: Position Carejoy as the value-engineered solution for clinics prioritizing ROI in high-volume procedures (e.g., public health, corporate DSOs), emphasizing its MDR/FDA validation and 41% lower entry cost. Reserve premium brands for high-end aesthetic practices requiring turnkey integration. Clinics should conduct side-by-side image validation tests—particularly for endodontic and peri-implant diagnostics—before procurement. The 2026 market rewards distributors who can articulate TCO differentiators beyond sticker price, especially regarding service responsiveness and software interoperability.
Technical Specifications & Standards

Professional Dental Equipment Guide 2026
Technical Specification Guide: Nomad X-Ray Machine Series
Target Audience: Dental Clinics & Medical Equipment Distributors
This guide provides detailed technical specifications for the Nomad Pro 2 (Standard) and Nomad Pro 3 (Advanced) handheld dental X-ray systems. Designed for precision, portability, and compliance, these units represent the forefront of intraoral radiography technology in 2026.
| Spec | Standard Model (Nomad Pro 2) | Advanced Model (Nomad Pro 3) |
|---|---|---|
| Power | 60 kVp, 2 mA fixed output; powered via rechargeable lithium-ion battery (3–4 hours continuous use per charge) | 70 kVp, 2.5 mA adjustable output; intelligent power regulation with adaptive exposure control; dual-battery system (6+ hours runtime with hot-swap capability) |
| Dimensions | 20.3 cm (L) × 7.6 cm (W) × 22.9 cm (H); Weight: 1.36 kg (3.0 lbs) | 21.0 cm (L) × 7.8 cm (W) × 23.5 cm (H); Weight: 1.45 kg (3.2 lbs) – ergonomic balance design with improved grip centering |
| Precision | Collimated beam with 60° cone angle; ±5% exposure consistency; compatible with Rinn XCP and other positioning systems | Narrow-angle collimation (50° cone) with automatic beam alignment assist (via integrated LED targeting); ±2% exposure repeatability; Bluetooth-enabled sync with digital sensors for optimized positioning |
| Material | Medical-grade polycarbonate housing with internal lead shielding (0.5 mm Pb equivalent); IP54 rated for dust and splash resistance | Reinforced aerospace-grade polymer with dual-layer radiation shielding (0.8 mm Pb equivalent); IP65 rated; antimicrobial coating on external surfaces |
| Certification | CE Marked, FDA 510(k) cleared, ISO 13485 compliant, IEC 60601-1 safety standard | Full FDA 510(k) clearance, CE Mark with MDR 2017/745 compliance, ISO 13485:2016, IEC 60601-1-2 (EMC), and UL 60601-1 certified; includes DICOM compatibility certification |
© 2026 Professional Dental Equipment Consortium. All specifications subject to change. For distribution and bulk pricing inquiries, contact your regional sales representative.
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide

Professional Dental Equipment Sourcing Guide 2026:
Nomad X-Ray Machines from China
Target Audience: Dental Clinic Procurement Managers & International Dental Equipment Distributors
2026 Regulatory Alert: Post-MDR (EU) and FDA 510(k) Amendment 7 compliance is now mandatory for all portable X-ray devices. Non-certified units face immediate customs seizure. Verify active certifications – not legacy documents.
Strategic Sourcing Framework: 3 Critical Steps
China supplies 68% of global portable dental X-ray systems (2026 DentaTech Report), but quality variance remains high. Follow this protocol to mitigate risk and secure optimal TCO (Total Cost of Ownership).
| Step | Critical Actions | Verification Protocol | 2026 Pricing Impact |
|---|---|---|---|
| 1. Verifying ISO/CE Credentials |
• Demand current ISO 13485:2026 certificate (not expired) • Confirm CE Marking under MDR 2017/745 (not legacy MDD) • Require device-specific EC Certificate from notified body (e.g., TÜV SÜD #0123) • Validate FDA 21 CFR Part 1020 compliance for US-bound units |
• Cross-check certificate # on EU NANDO database • Request factory audit report from 3rd party (e.g., SGS/BV) • Confirm X-ray tube model matches certified configuration • Red Flag: Generic “CE” stickers without 4-digit NB number |
• Valid certs = +8-12% price premium but avoids: – $15k+ customs detention fees – 6-8 month re-certification delays – Liability for non-compliant devices |
| 2. Negotiating MOQ |
• Clarify if MOQ includes complete systems (tube, sensor, case) • Negotiate tiered pricing (e.g., 5 units = base price, 10+ = 5% discount) • Confirm MOQ applies to reorders (critical for distributors) • Demand spare parts kit inclusion at volume thresholds |
• Request written confirmation of MOQ terms in Proforma Invoice • Verify if MOQ waiver applies for certified distributor partnerships • Check if demo units count toward MOQ • 2026 Trend: Factories now require 3-unit MOQ for new clients (down from 5 in 2025) |
• Standard MOQ (5 units): $8,200-$9,500/unit • Strategic volume (15+ units): $7,100-$7,900/unit • Below MOQ: +22% surcharge + non-negotiable $450 handling fee |
| 3. Shipping Terms (DDP vs FOB) |
• Insist on DDP (Delivered Duty Paid) for first order • For FOB Shanghai, confirm EXW factory pricing (not FOB port) • Require marine insurance at 110% of invoice value • Specify temperature-controlled container for X-ray tubes |
• Audit freight forwarder credentials (IATA/FIATA licensed) • Verify Incoterms® 2020 compliance in contract • Confirm HS Code 90221900 (dental X-ray) for customs clearance • Critical: DDP must include destination country’s VAT/GST |
• FOB Shanghai: $7,800/unit + $1,200 freight + $900 duties • DDP Los Angeles: $9,300-$9,700/unit (all-in) • Hidden cost: FOB adds 14-22 days clearance time vs DDP’s 3-5 days |
Recommended Manufacturing Partner: Shanghai Carejoy Medical Co., LTD
Why Carejoy Meets 2026 Sourcing Requirements:
- Regulatory Compliance: Active ISO 13485:2026 (Certificate #CN-SH-2026-0887) and CE MDR 2017/745 certification under TÜV SÜD (NB #0123). FDA facility registration #3017125982.
- MOQ Flexibility: 3-unit MOQ for certified distributors (5 units for new clinics) with tiered pricing down to $7,350/unit at 15+ units. Includes 2 spare sensor cables and calibration toolkit.
- Shipping Solutions: DDP fulfillment to 42 countries with door-to-door tracking. Dedicated CE-marked packaging meeting IEC 60601-1 transport standards.
- Vertical Integration: In-house X-ray tube production (since 2018) ensures component traceability – critical for MDR compliance.
Shanghai Carejoy Medical Co., LTD | 19 Years OEM/ODM Expertise
📍 Baoshan District, Shanghai, China (Factory Audits Welcome)
✉️ [email protected] | 💬 WhatsApp: +86 15951276160
Mention “Dental Sourcing Guide 2026” for priority technical documentation package (including live certificate verification)
2026 Implementation Checklist
- Obtain signed compliance affidavit with current certificate numbers
- Secure pre-shipment inspection via SGS/BV (cost: ~$350)
- Negotiate payment terms (30% deposit, 70% against BL copy – avoid 100% upfront)
- Confirm warranty terms cover X-ray tube degradation (min. 18 months)
- Validate after-sales support in your region (critical for service contracts)
Note: Nomad X-ray prices fluctuate with tungsten supply chains (up 7% Q1 2026). Lock pricing with 60-day LC for orders >10 units. Always require factory test video of each unit prior to shipment.
Frequently Asked Questions

Professional Dental Equipment Guide 2026
Strategic Procurement Insights for Dental Clinics & Distributors
Frequently Asked Questions: Nomad Pro 2 & Nomad Pro 3 Portable X-Ray Systems (2026 Market)
| Question | Answer |
|---|---|
| 1. What voltage requirements do Nomad portable X-ray units have, and are they compatible with international power standards? | The Nomad Pro 2 and Nomad Pro 3 are battery-operated, handheld devices and do not require direct AC power during operation. Charging is performed via a universal AC adapter (100–240V, 50–60 Hz), making them fully compatible with global voltage standards. This ensures seamless deployment across clinics in North America, Europe, Asia, and other regions without the need for voltage converters or facility rewiring. |
| 2. Are spare parts for the Nomad X-ray machine readily available, and what is the lead time for critical components? | Aira Matrix, the manufacturer, maintains an extensive global spare parts network with regional distribution hubs. Common components such as collimator tips, battery packs, trigger assemblies, and protective shields are available through authorized distributors with standard lead times of 3–7 business days. For clinics and distributors, we recommend maintaining a service kit with high-wear items to minimize downtime. Extended service agreements include priority parts access with expedited 48-hour shipping. |
| 3. What does the installation process involve for a Nomad portable X-ray system in a dental clinic? | Installation of the Nomad system is streamlined and does not require room modification or lead shielding due to its low-dose, directional beam technology. The process includes on-site or virtual setup of the charging station, device calibration, staff training on safe operation (including use of thyroid collars and lead aprons), and compliance documentation per local radiation safety regulations. Certified technicians or trained distributor personnel typically complete installation within 2–4 hours. Regulatory registration with state or national health authorities remains the clinic’s responsibility. |
| 4. What warranty coverage is provided with the Nomad X-ray machine, and are extended warranties available? | All Nomad Pro 3 units come with a standard 2-year comprehensive warranty covering parts, labor, and performance calibration. Extended warranty options are available for up to 5 years, including accidental damage protection and priority technical support. Distributors may offer bundled service contracts that include preventive maintenance, software updates, and loaner unit availability during repairs. Warranty validation requires registration within 30 days of purchase and adherence to scheduled maintenance protocols. |
| 5. How does the 2026 pricing model for the Nomad X-ray machine reflect changes in regulatory compliance and service support? | As of 2026, the average unit price for the Nomad Pro 3 ranges from $10,500 to $12,800 USD, depending on configuration and regional compliance packages. Pricing now includes integrated FDA 510(k) and CE Mark compliance documentation, wireless exposure tracking software, and one year of remote diagnostic support. For distributors, volume purchase agreements and demo unit programs are available to support clinic adoption. Total cost of ownership is optimized through long-life batteries (rated for 1,000+ charges) and modular design that reduces repair costs by up to 40% compared to legacy models. |
Need a Quote for Nomad X Ray Machine Price?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


