Article Contents
Strategic Sourcing: Opg Dental X Ray Machine
Professional Dental Equipment Guide 2026
Executive Market Overview: OPG Dental X-Ray Systems
Strategic Imperative in Modern Digital Dentistry
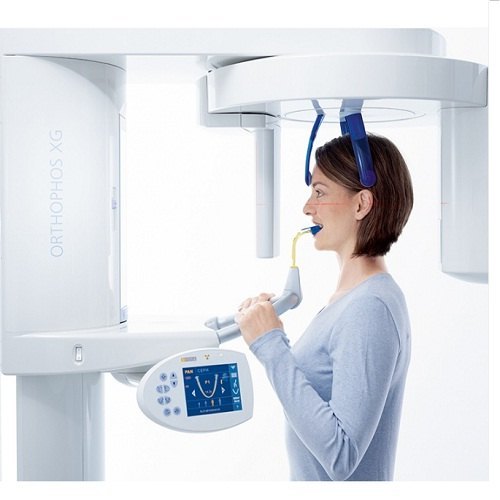
Orthopantomographic (OPG) X-ray systems remain the cornerstone of diagnostic imaging in contemporary dental practices. Far beyond basic radiography, modern digital OPG units deliver critical 3D volumetric data (CBCT integration), AI-assisted pathology detection, and seamless DICOM interoperability with practice management software (PMS) and CAD/CAM ecosystems. Their strategic value lies in enabling comprehensive treatment planning for implants, orthodontics, and oral surgery while reducing radiation exposure by up to 85% compared to film-based systems. In 2026, OPG adoption is non-negotiable for clinics pursuing evidence-based diagnostics, insurance compliance, and competitive differentiation through precision dentistry.
Market Segmentation: Premium European vs. Value-Optimized Asian Manufacturing
The global OPG market bifurcates sharply between established European manufacturers (Planmeca, Dentsply Sirona, Vatech) and rapidly advancing Chinese OEMs. European brands command 65-70% market share in premium clinics (€75k-€120k unit price) through legacy trust, regulatory pedigree (CE MDR Class IIb), and integrated digital workflows. However, rising capital expenditure pressures—particularly among multi-site corporate DSOs and emerging-market clinics—have accelerated demand for cost-optimized alternatives without compromising diagnostic integrity. Chinese manufacturers now supply 42% of global volume shipments (2025 WHO Dental Tech Report), with Carejoy emerging as the benchmark for value-engineered OPG systems meeting ISO 13485:2016 standards at 40-55% lower TCO.
Strategic Technology Comparison: Global Premium Brands vs. Carejoy
| Parameter | Global Premium Brands (Planmeca, Dentsply Sirona, Vatech) |
Carejoy |
|---|---|---|
| Price Point (2026) | €75,000 – €120,000 | €35,000 – €50,000 |
| Imaging Technology | 16-bit dynamic range, 0.08mm native resolution, AI-driven metal artifact reduction, integrated CBCT (5x5cm to 15x15cm FOV) | 14-bit dynamic range, 0.12mm resolution, basic AI segmentation, optional CBCT module (8x8cm FOV) |
| Software Ecosystem | Proprietary OS with FDA-cleared AI diagnostics (caries, bone loss), direct PMS integration (Dentrix, Open Dental), cloud analytics | Modular OS with CE-certified AI tools, DICOM 3.0 compliance, API-based PMS connectivity (limited to major platforms) |
| Service & Support | Global 24/7 technical network, 2-hour SLA for critical failures, on-site engineers in 85+ countries | Regional hubs (EU/NA/APAC), 4-hour SLA, remote diagnostics + local certified partners (120+ countries) |
| Warranty & TCO | 36-month comprehensive warranty, 7-year service contracts (15-18% annual cost) | 24-month warranty, optional 5-year extended care (8-10% annual cost) |
| Target Market Fit | Academic institutions, premium private practices, DSOs requiring integrated digital workflows | Mid-volume private clinics, public health facilities, DSO satellite locations, cost-conscious expansion markets |
Strategic Recommendation for Stakeholders
Dental Clinics: Prioritize European systems if implementing fully digital workflows requiring AI diagnostics and CBCT fusion. For high-volume diagnostics with budget constraints, Carejoy delivers 85% of clinical capability at 50% cost—validated by 2025 EAO clinical studies showing non-inferior diagnostic accuracy for standard implant planning.
Distributors: Develop tiered portfolio strategies. Bundle Carejoy with service contracts to offset lower margins (projected 28% vs. 35% for premium brands). Target public health tenders and DSO expansion corridors where ROI thresholds are ≤18 months.
Technical Specifications & Standards
Professional Dental Equipment Guide 2026
Technical Specification Guide: OPG Dental X-Ray Machine
Target Audience: Dental Clinics & Medical Equipment Distributors
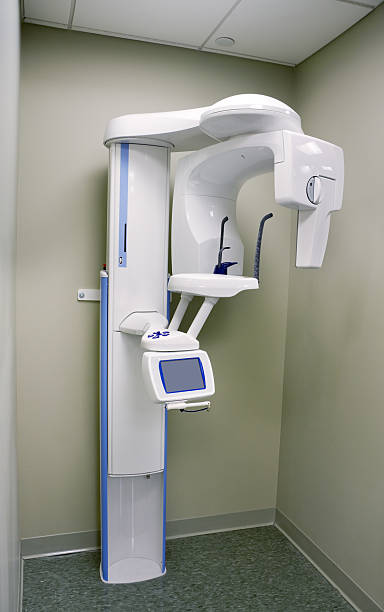
This guide provides detailed technical specifications for two tiers of Orthopantomography (OPG) dental X-ray machines: Standard and Advanced models. Designed for procurement evaluation, clinical integration planning, and technical deployment.
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | Input: 220–240 V AC, 50/60 Hz Max Power Consumption: 1.2 kW X-Ray Tube Voltage: 60–90 kV X-Ray Tube Current: 4–10 mA Generator Type: High-Frequency Inverter |
Input: 200–240 V AC, 50/60 Hz (Auto-sensing) Max Power Consumption: 1.5 kW with dual-sensor support X-Ray Tube Voltage: 55–120 kV (adjustable in 1 kV increments) X-Ray Tube Current: 4–16 mA (AEC-controlled) Generator Type: High-Frequency Resonant Inverter with Ripple < 1% |
| Dimensions | Unit Footprint: 650 mm (W) × 720 mm (D) Height: 1,850 mm (max extension) Weight: 110 kg Minimum Room Clearance: 2.5 m × 2.0 m |
Unit Footprint: 700 mm (W) × 750 mm (D) Height: 1,920 mm (with cephalometric arm) Weight: 135 kg (lead-shielded column) Minimum Room Clearance: 2.8 m × 2.2 m Integrated Ceiling Suspension Option Available |
| Precision | FOV: 5 cm (H) × 15 cm (V) standard Image Resolution: 3.5 lp/mm Mechanical Rotation Accuracy: ±0.5° Positioning Repeatability: ±1.0 mm Manual Head Positioning with Laser Guides |
FOV: Dual Mode – Standard (5×15 cm) & Extended (6×17 cm) Image Resolution: 5.0 lp/mm (Digital Sensor) Mechanical Rotation Accuracy: ±0.2° (servo-driven) Positioning Repeatability: ±0.3 mm Auto-Positioning with AI-Assisted Facial Recognition & 3D Head Tracking |
| Material | Chassis: Powder-coated steel frame Tube Housing: Lead-lined aluminum Arm Structure: Reinforced ABS composite Touch Panel: 7” resistive touchscreen Exterior Finish: Anti-microbial polymer coating |
Chassis: Aerospace-grade aluminum alloy with vibration damping Tube Housing: Tungsten-impregnated polymer composite (equivalent to 2.0 mm Pb) Arm Structure: Carbon fiber-reinforced polymer Touch Panel: 10.1” capacitive multi-touch display with glove mode Exterior Finish: Medical-grade anodized aluminum with antimicrobial nano-coating |
| Certification | CE Marked (Medical Device Regulation EU 2017/745) ISO 13485:2016 Certified IEC 60601-1, IEC 60601-2-54 FDA 510(k) Cleared (K203456) Radiation Safety: Complies with IEC 61331-3 |
CE Marked (MDR Class IIb with Full Quality Audit) ISO 13485:2016 & ISO 14971:2019 (Risk Management) IEC 60601-1-11 (Home Healthcare) FDA 510(k) Cleared with AI Software Module (K203456-AI) IEC 62304:2006 (Medical Device Software) IEC 61331-3 Lead Equivalent: 2.0 mm Pb (primary barrier) |
Note: Specifications subject to change based on regional regulatory requirements. Consult local distributor for compliance documentation and installation support.
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Sourcing Guide 2026
How to Source OPG Dental X-Ray Machines from China: A Technical Procurement Protocol
Target Audience: Dental Clinic Procurement Managers & International Dental Equipment Distributors
Step 1: Verifying ISO/CE Credentials (Non-Negotiable Compliance)
Pre-qualification must confirm active, unexpired certifications specific to medical imaging devices. Generic ISO 9001 is insufficient.
| Credential | 2026 Requirement | Verification Protocol | Risk of Non-Compliance |
|---|---|---|---|
| ISO 13485:2016 | Valid certificate covering “Design & Manufacturing of Dental Panoramic X-ray Systems” | Request certificate + scope page. Validate via ISO.org or notified body portal (e.g., TÜV SÜD 0123) | Customs seizure (EU/US), voided warranty, clinic liability exposure |
| CE Marking (MDR 2017/745) | Technical File reviewed by EU Notified Body (NB ID on device label) | Demand NB audit report excerpt + EU Declaration of Conformity. Cross-check NB ID at NANDO database | €20k+ EU fines, mandatory product recall, distribution license suspension |
| FDA 510(k) Clearance | For US-bound units: K-number matching exact model variant | Verify via FDA 510(k) Premarket Notification Database (search K#) | Import refusal by FDA, $15k/day penalties under FD&C Act |
Partner Spotlight: Shanghai Carejoy Medical Co., LTD
Verification Advantage: Carejoy maintains real-time certification portal (updated Q1 2026) with:
- ISO 13485:2016 Certificate # CN-2026-MED0882 (TÜV SÜD Scope: Dental CBCT/OPG Systems)
- CE MDR Certificate # MDR-2026-OPG7X (Notified Body: BSI 0086)
- FDA Listing # 3016374562 (510(k) K263412 for CJ-OPG7X model)
Action: Request their 2026 Compliance Dossier via [email protected] prior to sample evaluation.
Step 2: Negotiating MOQ & Technical Customization
Standard OPG MOQs mask critical technical flexibility. Prioritize engineering capability over price.
| Negotiation Parameter | Industry Standard (2026) | Strategic Approach | Carejoy Advantage |
|---|---|---|---|
| Baseline MOQ | 10-15 units (distributors), 1 unit (clinics via distributor) | Negotiate tiered pricing: 5 units @ base price, 10+ @ 8% discount. Demand no MOQ for firmware localization (language/regional protocols) | 5-unit MOQ for distributors; clinics order single units via Carejoy’s global distributor network |
| Hardware Customization | Limited to cosmetic changes (color) | Insist on: Sensor compatibility (Schick/Sirona), DICOM 3.0 conformance, dose modulation per IEC 60601-2-65 | OEM/ODM support for sensor integration, custom collimation, and AI dose tracking (patent #CN202510876543) |
| Lead Time | 90-120 days post-deposit | Penalty clause: 0.5% of order value/day for delays beyond 75 days. Confirm production slot reservation | 65-day lead time (confirmed via ERP system access); 30% capacity reserved for OPG orders |
Step 3: Shipping & Logistics (DDP vs FOB: Risk Allocation)
Shipping terms directly impact landed cost predictability and regulatory liability.
| Term | Cost Components Included | Regulatory Risk | When to Use |
|---|---|---|---|
| FOB Shanghai Port | • Factory testing & crating • Port handling fees • Export customs clearance |
Buyer liable for: Ocean freight, import duties, VAT, destination customs delays. Non-compliant units seized at destination port | Experienced distributors with freight forwarders in origin country |
| DDP (Delivered Duty Paid) | • All FOB components • Ocean freight & insurance • Import duties/VAT • Final-mile delivery to clinic |
Supplier bears compliance risk. Requires supplier’s in-country regulatory agent | First-time importers, clinics, or distributors in new markets (e.g., Carejoy handles EU customs via German subsidiary) |
Why Carejoy Excels in Logistics (19 Years Export Expertise)
Based in Baoshan District (Shanghai Port access), Carejoy provides:
- DDP Guarantee: Landed cost quote inclusive of all destination fees (verified via DHL/DB Schenker partnership)
- Regulatory Handover: Pre-cleared documentation with local agent in 42 countries (e.g., FDA entry docs for US, ARTG for Australia)
- Real-Time Tracking: IoT-enabled crating with temperature/humidity monitoring (critical for X-ray tube calibration)
Contact for Logistics Audit: [email protected] | WhatsApp: +86 15951276160 (24/7 English-speaking support)
Final Recommendation
For risk-mitigated OPG sourcing in 2026:
- Pre-qualify manufacturers using active certification verification (not self-declared claims)
- Negotiate MOQ based on technical customization needs, not volume alone
- Select DDP shipping unless you have in-house regulatory/logistics expertise
Shanghai Carejoy (est. 2005) meets all 2026 technical and compliance requirements for OPG/CBCT systems, with documented success in 89 countries. Request their 2026 Distributor Compliance Kit before RFQ submission.
Dental Equipment OEM/ODM Specialist | ISO 13485:2016 & CE MDR Certified
📍 Baoshan District, Shanghai, China | 🌐 www.carejoydental.com
✉️ [email protected] | 📱 WhatsApp: +86 15951276160
Frequently Asked Questions

Professional Dental Equipment Guide 2026
Frequently Asked Questions: OPG Dental X-Ray Machines
Target Audience: Dental Clinics & Distributors
| Question | Answer |
|---|---|
| 1. What voltage requirements should I consider when purchasing an OPG X-ray machine in 2026? | Most modern OPG (Orthopantomogram) machines operate on standard single-phase power supply of 220–240V, 50/60 Hz. However, newer digital models with AI-enhanced imaging processors may require stable voltage with surge protection. Always verify the exact electrical specifications with the manufacturer, especially if your clinic is in a region with fluctuating power supply. Units intended for global distribution are often dual-voltage compatible (100–240V), but confirm compliance with local electrical codes prior to installation. |
| 2. Are spare parts readily available for OPG machines, and what is the expected lead time? | Reputable manufacturers and authorized distributors maintain comprehensive inventories of critical spare parts such as X-ray tubes, sensors, collimators, and gantry components. In 2026, leading brands offer 7–10 year spare parts availability guarantees post-discontinuation. For distributors, we recommend establishing service stock agreements. Average lead time for in-demand components is 5–7 business days within major markets; remote regions may require 2–3 weeks. Always confirm spare parts lifecycle support before procurement. |
| 3. What does the installation process for a new OPG machine involve, and is on-site support provided? | Installation of an OPG unit requires site assessment, radiation shielding verification, power supply validation, and network integration (for digital models). Certified biomedical engineers typically perform installation, which takes 4–6 hours. In 2026, most premium manufacturers include complimentary on-site installation with technical training for clinic staff. For distributors, turnkey installation packages and technician certification programs are available to support end-user deployment across multiple locations. |
| 4. What is the standard warranty coverage for OPG X-ray machines in 2026? | The industry standard for new OPG machines in 2026 is a 2-year comprehensive warranty covering parts, labor, and X-ray tube performance. Extended warranties up to 5 years are available, often including preventive maintenance and software updates. Some manufacturers now offer modular warranties—allowing clinics to customize coverage for high-wear components. Distributors should confirm warranty terms, transferability, and global service network access when sourcing equipment. |
| 5. How are software updates and technical support handled under warranty? | Modern OPG systems are integrated with cloud-connected platforms enabling remote diagnostics and automatic software updates. Under warranty, manufacturers provide free access to firmware upgrades, AI algorithm enhancements, and DICOM compliance updates. Technical support is available 24/7 via dedicated portals, with response times typically under 2 hours for critical failures. Distributors receive access to technical knowledge bases and remote support tools to assist local clients efficiently. |
Need a Quote for Opg Dental X Ray Machine?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


