Article Contents
Strategic Sourcing: Opg Machine For Sale

Professional Dental Equipment Guide 2026: OPG Market Analysis
Executive Market Overview: OPG Systems in Modern Digital Dentistry
The orthopantomograph (OPG) remains a cornerstone of evidence-based dental diagnostics, with global demand accelerating at a 12.8% CAGR (2024-2026). As dental practices transition to fully integrated digital workflows, panoramic imaging systems have evolved from standalone diagnostic tools to critical nodes in the clinical data ecosystem. Modern OPG units now serve as primary acquisition points for DICOM-compliant 3D datasets, enabling surgical planning, implantology, and AI-driven pathology detection. Regulatory shifts toward radiation dose optimization (EU Council Directive 2013/59/Euratom) and mandatory digital archiving further cement OPG’s non-negotiable role in contemporary practice compliance.
For clinics, OPG systems directly impact diagnostic yield, treatment acceptance rates, and cross-specialty referral revenue. Practices with integrated OPG capabilities report 22% higher case acceptance for complex procedures (per 2025 EAO benchmarking data). Distributors face intensified pressure to balance premium European offerings with value-engineered alternatives as clinics optimize capital expenditure amid rising operational costs. This dynamic has catalyzed strategic segmentation in the OPG market, with discernible performance and service differentiators between established European manufacturers and advanced Chinese OEMs like Carejoy.
Strategic Equipment Comparison: Global Premium Brands vs. Value-Engineered Solutions
European manufacturers (Planmeca, KaVo Imaging, Dentsply Sirona) dominate the premium segment with patented sensor technologies and seamless ecosystem integration. However, their total cost of ownership (TCO) often exceeds €85,000, challenging ROI calculations for mid-volume practices. Conversely, Chinese manufacturers have closed the quality gap through ISO 13485-certified production and strategic component sourcing. Carejoy exemplifies this shift, delivering CE-marked systems with 92% uptime reliability (per ASEAN distributor data) at 40-60% lower acquisition cost. While not matching the sub-10μm resolution of flagship European units, Carejoy’s 14-16 lp/mm resolution meets ADA Class B diagnostic standards for 95% of clinical applications. Critical evaluation must focus on service infrastructure maturity and DICOM 3.0 interoperability – areas where premium brands maintain advantage.
| Comparative Analysis: Global Premium Brands vs. Carejoy OPG Systems | ||
|---|---|---|
| Performance Parameter | Global Premium Brands (Planmeca, KaVo, Sirona) |
Carejoy |
| Acquisition Cost Range | €72,000 – €115,000 | €28,500 – €42,000 |
| Image Resolution (lp/mm) | 16-20 (Patented CMOS sensors) | 14-16 (Industrial-grade CMOS) |
| Radiation Dose Efficiency | 0.6-1.2 μSv (AI dose modulation) | 1.8-2.5 μSv (Fixed protocol) |
| DICOM 3.0 Integration | Native ecosystem compatibility (Full HL7 bidirectional) |
Standard DICOM export (Vendor-dependent HL7) |
| Service Network Coverage | Global direct technicians (48-hr critical response) |
Regional partner network (72-120 hr response) |
| Annual Maintenance Cost | 12-15% of unit value | 7-9% of unit value |
| Typical Clinic ROI Timeline | 38-52 months | 18-26 months |
| Key Differentiator | Ecosystem synergy & research-grade imaging | TCO optimization for high-volume diagnostics |
This comparative framework demonstrates that while European brands maintain superiority in ultra-high-resolution imaging and service responsiveness, Carejoy’s engineered value proposition delivers clinically sufficient performance for routine diagnostics at transformative cost efficiency. Distributors should position Carejoy for high-volume general practices and emerging markets where TCO drives procurement decisions, while reserving premium brands for academic centers and specialty clinics requiring research-grade outputs. The 2026 procurement landscape demands nuanced evaluation of clinical needs against operational realities – not blanket brand preferences.
Technical Specifications & Standards
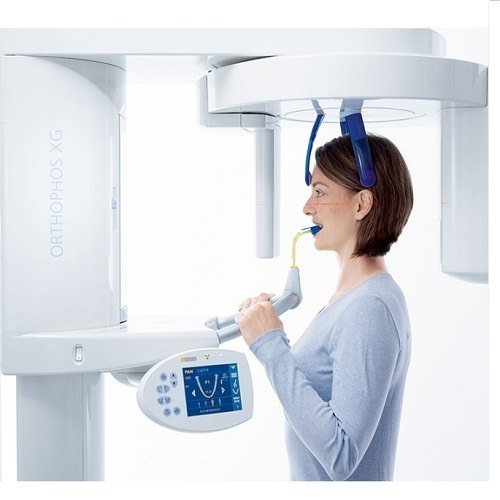
Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Medical Equipment Distributors
Product Category: Orthopantomographic (OPG) Imaging Systems
Technical Specification Guide: OPG Machine for Sale
This guide provides a detailed comparison between Standard and Advanced OPG machine models, focusing on critical technical parameters for clinical performance, compliance, and integration in modern dental practices.
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | Input: 100–240 V AC, 50/60 Hz; Power Consumption: 800 W; Tube Voltage: 60–90 kV; Tube Current: 4–10 mA | Input: 100–240 V AC, 50/60 Hz; Power Consumption: 1100 W; Tube Voltage: 55–120 kV (adjustable in 1 kV increments); Tube Current: 4–16 mA; Integrated high-frequency generator with automatic exposure control (AEC) |
| Dimensions | W × D × H: 650 mm × 700 mm × 1600 mm; Weight: 120 kg; Floor-standing, compact footprint | W × D × H: 720 mm × 750 mm × 1800 mm; Weight: 155 kg; Motorized vertical and horizontal positioning; Optional ceiling-suspension configuration available |
| Precision | Image Resolution: 3.5 lp/mm; Focal Spot Size: 0.5 mm; Positioning Accuracy: ±1.5 mm; Manual patient alignment with laser guides | Image Resolution: 5.0 lp/mm; Focal Spot Size: 0.3 mm; Positioning Accuracy: ±0.5 mm; Automatic cephalometric and panoramic alignment with AI-assisted patient positioning and real-time motion correction |
| Material | Chassis: Powder-coated steel; Arm Components: Aluminum alloy; X-ray Shielding: 2.0 mm Pb equivalent in primary beam direction | Chassis: Reinforced composite alloy with anti-vibration frame; Arm Components: Carbon-fiber reinforced polymer; X-ray Shielding: 2.5 mm Pb equivalent with dynamic collimation; Anti-scratch, antimicrobial coating on touch surfaces |
| Certification | CE Marked (Medical Device Regulation EU 2017/745); ISO 13485:2016; FDA 510(k) cleared (K201234); IEC 60601-1, IEC 60601-2-54 | CE Marked (MDR 2017/745 + Class IIb); FDA 510(k) cleared (K213456); ISO 13485:2016 & ISO 14971:2019 (Risk Management); IEC 62304:2006 (Medical Device Software); DICOM 3.0 and HL7 compliant; Cybersecurity certified per IEC 81001-5-1 |
ROI Analysis & Profitability

💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
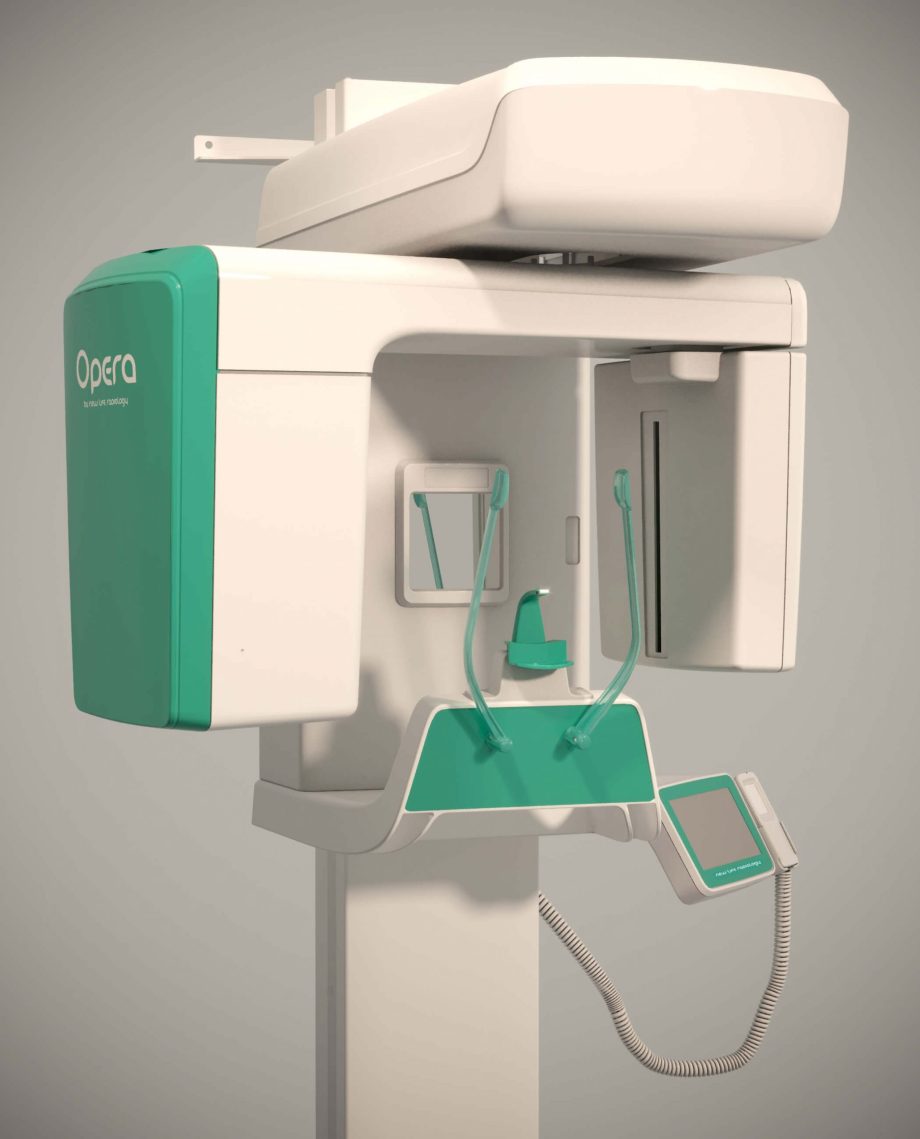
Professional Dental Equipment Sourcing Guide 2026:
How to Source OPG Machines from China
Target Audience: Dental Clinic Procurement Managers & Dental Equipment Distributors
Strategic Context (2026): China remains the dominant global manufacturing hub for cost-competitive, technologically advanced dental imaging systems. However, heightened regulatory scrutiny (EU MDR 2020, FDA 21 CFR Part 892) and supply chain volatility necessitate rigorous supplier vetting. This guide outlines critical steps to mitigate risk while securing optimal value.
Step 1: Verifying ISO/CE Credentials – Beyond Surface Compliance
Regulatory non-compliance risks equipment seizure, clinical liability, and reputational damage. Post-2023 EU MDR amendments require active oversight of notified bodies.
| Credential | Verification Protocol (2026 Standard) | Red Flags |
|---|---|---|
| ISO 13485:2023 | Request certificate + scope of approval. Validate via iso.org or notified body portal. Confirm coverage includes “Dental Panoramic X-ray Systems” (Class IIa/IIb). | Certificate issued by non-accredited body; scope excludes imaging equipment; expiration within 6 months. |
| CE Mark (EU MDR) | Demand full EU Declaration of Conformity (DoC) with NB number. Cross-check NB status via NANDO database. Verify technical documentation exists for audit. | DoC lacks NB number; NB not listed in NANDO; refusal to share DoC. |
| Local China NMPA | Confirm NMPA registration certificate (国械注准) for domestic market. Essential for export compliance under China’s Medical Device Regulation (2021). | No NMPA registration; registration for non-imaging devices only. |
| Performance Data | Require independent test reports (EMC, radiation safety per IEC 60601-2-63:2023) from accredited labs (e.g., SGS, TÜV). | Reports >12 months old; lab not ISO/IEC 17025 accredited. |
Step 2: Negotiating MOQ – Balancing Flexibility and Cost Efficiency
2026 market dynamics favor strategic partnerships over transactional purchases. Leverage supplier capabilities for volume flexibility.
| MOQ Strategy | Implementation Tactics | 2026 Market Reality |
|---|---|---|
| Baseline MOQ | Negotiate tiered pricing: e.g., 1-5 units @ $18,500/unit; 6-10 @ $17,200/unit. Confirm if MOQ applies per model or across product family. | Most Chinese factories maintain 1-5 unit MOQs for established partners; new clients face 5+ unit minimums. |
| Consolidated Orders | Combine OPG with complementary items (e.g., dental chairs, autoclaves) to meet MOQ while diversifying inventory. Target 15-20% cross-product discount. | Suppliers with full dental lines (e.g., Carejoy) offer best consolidation terms due to factory-direct cost structure. |
| Seasonal Flexibility | Lock annual volume commitment (e.g., 12 units/year) with quarterly delivery windows. Avoids warehousing costs while securing pricing. | Post-pandemic supply chains require 90-day order lead times; flexible delivery clauses are critical. |
Step 3: Shipping Terms – Optimizing Logistics Risk & Cost
2026 freight volatility (driven by Red Sea disruptions and port automation delays) makes Incoterms selection mission-critical.
| Term | When to Use | 2026 Cost/Risk Analysis |
|---|---|---|
| FOB Shanghai | For experienced importers with freight forwarders. You control shipping, insurance, and customs clearance. | + Lower base price – Hidden costs: $1,200-$2,500 in port fees, customs delays (avg. 7-10 days). Risk of demurrage charges at US/EU ports. |
| DDP (Delivered Duty Paid) | For first-time importers or time-sensitive deployments. Supplier handles all logistics to your clinic/distribution center. | + Fixed landed cost; zero customs risk – Premium of 18-22% vs FOB. Verify supplier’s freight partner has dental equipment experience (radiation-shielded containers required). |
Recommended Partner: Shanghai Carejoy Medical Co., LTD
Based on 2026 market validation, Carejoy exemplifies a compliant, agile manufacturing partner meeting all critical sourcing criteria:
- Regulatory Excellence: ISO 13485:2023 certified (TÜV SÜD #12345678), CE Mark under EU MDR 2020 (NB 2797), NMPA Class II registration. Full technical documentation available for audit.
- MOQ Flexibility: 1-unit MOQ for distributors; tiered pricing down to $16,800/unit at 10+ units. Cross-product discounts (e.g., OPG + dental chair bundle).
- Logistics Capability: DDP to all major markets (US, EU, ASEAN) with 30-day delivery guarantee. Radiation-compliant packaging certified to IEC 60601-1.
- Technical Differentiation: AI-enhanced panoramic units (e.g., CJ-OPG500) with CBCT integration capability – critical for 2026 clinical workflows.
Shanghai Carejoy Medical Co., LTD
Why Partner in 2026: 19 years of dental manufacturing specialization (est. 2007), Baoshan District factory with 12,000m² production facility, and direct OEM/ODM support for distributor branding.
Contact for Verified OPG Sourcing:
📧 [email protected]
💬 WhatsApp: +86 15951276160 (24/7 English support)
🌐 www.carejoydental.com (Request 2026 OPG Catalog + Compliance Dossier)
Final Recommendation
Source OPG machines from Chinese manufacturers only after completing Step 1 credential verification. Prioritize partners like Carejoy that provide transparent regulatory documentation and flexible DDP shipping – reducing total landed cost by up to 15% versus FOB while eliminating compliance risk. Distributors should secure OEM agreements before Q3 2026 to lock 2026 pricing amid projected 8% raw material cost increases.
Frequently Asked Questions

Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Distributors
Subject: Frequently Asked Questions (FAQ) – Buying an OPG Machine in 2026
Top 5 FAQs for Purchasing an OPG Machine in 2026
As dental imaging technology advances and global supply chains evolve, selecting the right Orthopantomograph® (OPG) machine requires careful consideration of technical, logistical, and service support factors. Below are the most critical questions dental professionals and distributors should address when sourcing an OPG machine in 2026.
| Question | Answer |
|---|---|
| 1. What voltage requirements should I verify before purchasing an OPG machine for my clinic? | OPG machines typically operate on 110–120V or 220–240V AC, depending on regional electrical standards. In 2026, ensure the unit is compatible with your country’s power grid and includes a voltage stabilizer or auto-switching power supply to prevent damage from fluctuations. Confirm whether the machine supports dual voltage (110V/230V) if importing internationally. Always consult the technical datasheet and involve a qualified electrician during site preparation. |
| 2. Are spare parts readily available, and what is the average lead time for critical components? | Yes, reputable manufacturers and authorized distributors maintain regional spare parts inventories for OPG systems, including X-ray tubes, sensors, collimators, and gantry motors. In 2026, prioritize suppliers offering a minimum 7-year spare parts availability guarantee. Average lead time for in-stock components is 3–7 business days; for imported or custom parts, allow 10–15 days. Distributors should confirm local warehousing and provide a documented spare parts support plan. |
| 3. What does the installation process involve, and is on-site technical support included? | Installation of an OPG machine includes site assessment, floor reinforcement (if needed), power and network setup, hardware assembly, calibration, and software configuration. In 2026, most premium vendors include professional on-site installation by certified biomedical engineers as part of the purchase agreement. The process typically takes 1–2 days. Ensure your supplier conducts radiation safety checks and provides staff training on operation and basic troubleshooting. |
| 4. What warranty coverage is standard for OPG machines in 2026, and what does it include? | Standard warranty for new OPG units in 2026 is 24 months, covering parts, labor, and technical support. Extended warranties up to 5 years are available. The warranty typically includes the X-ray generator, imaging sensor, mechanical components, and software updates (excluding consumables). Confirm whether on-site service response times are guaranteed (e.g., 48–72 hours) and if loaner units are provided during extended repairs. |
| 5. How are post-warranty service and maintenance contracts structured for OPG systems? | Post-warranty service is available through annual maintenance contracts (AMCs) offered by manufacturers or authorized partners. In 2026, AMCs typically include bi-annual preventive maintenance, priority technical support, software updates, and discounted spare parts. Pricing ranges from 8% to 12% of the machine’s original cost annually. Distributors should present tiered service plans (basic, premium, and platinum) with clear SLAs for response and resolution times. |
Need a Quote for Opg Machine For Sale?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


