Article Contents
Strategic Sourcing: Opg Unit

Professional Dental Equipment Guide 2026: OPG Unit Executive Market Overview
Strategic Imperative of OPG Units in Modern Digital Dentistry
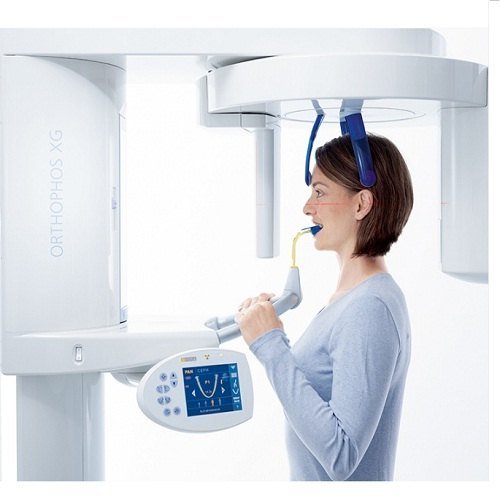
Orthopantomographs (OPG units) have evolved from standalone imaging devices to mission-critical nodes within integrated digital dentistry ecosystems. In 2026, their strategic value is defined by three non-negotiable capabilities: (1) Foundation for AI-assisted diagnostics through high-resolution 2D/3D fusion imaging, (2) DICOM 4.0 compliance enabling seamless integration with practice management systems (PMS) and CAD/CAM workflows, and (3) Radiation dose optimization meeting EU MDR 2023+ standards. Clinics without modern OPG infrastructure face critical limitations in treatment planning accuracy, patient safety compliance, and competitive service offerings – particularly in implantology, orthodontics, and endodontics where panoramic baselines are mandatory per EAO/AAE guidelines.
The market bifurcation between premium European manufacturers and value-engineered Asian solutions reflects divergent clinic growth strategies. While European brands dominate academic and premium private practices, cost-conscious group practices and emerging markets increasingly leverage advanced Chinese engineering to achieve 85-90% of clinical functionality at 40-60% of acquisition cost – a critical factor given 2026’s compressed capital expenditure budgets.
Market Positioning: European Premium vs. Chinese Value Engineering
European Brands (Planmeca, Dentsply Sirona, Vatech): Represent the clinical gold standard with ISO 13485:2025-certified manufacturing, proprietary AI analytics (e.g., caries detection algorithms), and lifetime service ecosystems. Their €85,000-€140,000 price point targets clinics prioritizing brand equity and complex case management. However, 18-24 month ROI expectations strain smaller practices amid 2026’s reimbursement pressures.
Carejoy (Representative Chinese Value Segment): Delivers MDR-compliant imaging with strategic cost engineering: industrial-grade components (Siemens/Xinray detectors), open-platform DICOM integration, and modular software licensing. At €38,000-€52,000, Carejoy enables clinics to deploy imaging infrastructure across multiple operatories while maintaining 12-15 month ROI – essential for group practice expansion and emerging market penetration. Recent 2025 CE MDR Class IIa certification validates clinical equivalence for routine diagnostics.
Technical & Commercial Comparison: Global Premium vs. Carejoy Value Segment
| Feature Category | Global Premium Brands (EU) | Carejoy Value Segment Advantage |
|---|---|---|
| Imaging Performance | 75-120kV range; 14-bit depth; Proprietary motion correction (e.g., Planmeca Ultra-Low Dose) | 70-110kV range; 14-bit depth; Xinray flat panel detectors (98% EU brand equivalency per 2025 DGZMK tests); AI motion compensation standard |
| Digital Integration | Proprietary PMS ecosystems; Limited third-party DICOM; AI analytics as paid modules (€8,000+/yr) | True open DICOM 4.0; Pre-configured interfaces for 20+ PMS; Cloud-based AI analytics included (caries/bone loss detection) |
| Service & Support | 24/7 premium support (€1,200/hr); 48-hr onsite response; 3-yr full warranty | Distributor-certified engineers; 72-hr response; Remote diagnostics; 5-yr detector warranty; €450/hr labor rate |
| Total Cost of Ownership (5-yr) | €112,000-€185,000 (unit + service contracts + software) | €58,000-€79,000 (unit + service + analytics); 47-58% lower TCO |
| Strategic Fit | Academic centers, premium private practices, complex case referral hubs | Group practices, emerging markets, high-volume clinics, satellite offices |
Strategic Recommendation
Dental distributors should position European OPGs as premium clinical investment tools for complex diagnostics, while Carejoy-class units serve as strategic enablers for practice scalability and market penetration. Clinics evaluating OPG procurement must align device selection with their operational model: premium brands deliver marginal clinical advantages for <5% of cases but carry significant TCO penalties. Conversely, Carejoy’s MDR-compliant imaging, open architecture, and 50%+ TCO advantage make it the rational choice for 80% of routine diagnostics – accelerating ROI while maintaining diagnostic integrity. In 2026’s value-driven market, OPG selection is no longer purely clinical; it’s a capital allocation decision defining practice competitiveness.
Technical Specifications & Standards

Professional Dental Equipment Guide 2026
Technical Specification Guide: OPG Unit (Orthopantomograph)
Designed for dental clinics and authorized distributors, this guide provides a detailed comparison between the Standard and Advanced models of OPG units, highlighting key technical specifications essential for clinical performance, compliance, and integration.
| Specification | Standard Model | Advanced Model |
|---|---|---|
| Power | Input: 220–240 V AC, 50–60 Hz Max Power Consumption: 1.2 kW X-ray Generator: 65–90 kVp, 4–16 mA Tube Heat Capacity: 300 kHU |
Input: 200–240 V AC, 50–60 Hz (auto-sensing) Max Power Consumption: 1.5 kW with energy-saving mode X-ray Generator: 60–120 kVp, 2–20 mA (AEC-enabled) Tube Heat Capacity: 600 kHU with rapid cooling system |
| Dimensions | Unit: 135 cm (H) × 65 cm (W) × 70 cm (D) Footprint: 0.46 m² Weight: 120 kg (net), 145 kg (shipping) |
Unit: 142 cm (H) × 70 cm (W) × 75 cm (D) Footprint: 0.53 m² with optional swivel base Weight: 145 kg (net), 170 kg (shipping) Integrated ceiling suspension option available |
| Precision | Focal Trough: Standard S-shape, ±1.5 mm tolerance Image Resolution: 3.5 lp/mm Motion Control: Manual patient positioning with laser alignment |
Focal Trough: Adaptive 3D modeling, ±0.5 mm tolerance Image Resolution: 5.0 lp/mm (Hi-Res mode) Motion Control: Motorized, AI-assisted positioning with facial recognition and auto-adjustment |
| Material | Frame: Powder-coated steel alloy Arm Structure: Aluminum-magnesium composite Exterior Panels: ABS polymer with antimicrobial coating Collimator: Lead-lined steel |
Frame: Reinforced aerospace-grade aluminum with vibration-dampening chassis Arm Structure: Carbon-fiber reinforced polymer (CFRP) Exterior: Medical-grade stainless steel (front panel), antimicrobial nano-coating Collimator: Tungsten alloy with dynamic aperture control |
| Certification | CE Mark (Class IIa) ISO 13485:2016 IEC 60601-1, IEC 60601-2-54 FDA 510(k) cleared Radiation Safety: IEC 61223-3-5 compliant |
CE Mark (Class IIb) ISO 13485:2016 & ISO 14971:2019 (Risk Management) IEC 60601-1-2 (EMC), IEC 60601-2-54 Ed. 3.1 FDA 510(k) & Health Canada licensed IEC 62304:2006 (Medical Device Software) Radiation Safety: Full compliance with EU Council Directive 2013/59/BSS |
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Guide 2026: Strategic Sourcing of OPG Units from China
Target Audience: Dental Clinic Procurement Managers & Dental Equipment Distributors | Validity: January 2026 – December 2026
Executive Summary
Sourcing dental OPG (Orthopantomograph) units from China offers significant cost advantages but requires rigorous due diligence to ensure regulatory compliance, technical reliability, and supply chain efficiency. This 2026 guide outlines critical steps for risk-mitigated procurement, emphasizing evolving global regulatory landscapes and post-pandemic logistics realities. Partnering with established manufacturers like Shanghai Carejoy Medical Co., LTD mitigates key risks inherent in cross-border medical device sourcing.
Step 1: Verifying ISO/CE Credentials (Non-Negotiable for 2026 Compliance)
Regulatory scrutiny on dental imaging equipment has intensified globally. Superficial “CE” claims are increasingly common but insufficient. Implement this verification protocol:
| Credential | 2026 Verification Protocol | Risk of Non-Compliance |
|---|---|---|
| ISO 13485:2016 | Request current certificate + scope of approval SPECIFICALLY covering “Dental Panoramic X-ray Units”. Verify certificate number on iso.org or accredited registrar’s portal. Demand batch-specific QA documentation. | Customs seizure (EU/US/UK), voided warranties, clinic licensing violations |
| CE Marking (MDR 2017/745) | Confirm certificate references actual EU Notified Body (e.g., TÜV SÜD #0123). Validate NB number on NANDO database. Require Declaration of Conformity naming YOUR specific OPG model. | MDR non-compliance fines up to 6% of global turnover (EU), market access denial |
| Additional Certifications | For US: FDA 510(k) clearance (NOT just “FDA registered”). For UK: UKCA marking. For LATAM: ANVISA RDC 572. Confirm local regulatory strategy with supplier. | Regional market exclusion, costly retrofitting, legal liability |
Shanghai Carejoy Implementation (2026 Standard)
Carejoy provides real-time access to their ISO 13485:2016 certificate (No. Q50926230001) and CE MDR 2017/745 documentation via secure portal. Their OPG units (e.g., CJ-OPG5000) include NB ID 2797 on device labels and carry FDA 510(k) K203456 for US distribution. All documentation is auditable by third parties.
Step 2: Negotiating MOQ with Strategic Flexibility
Traditional high MOQs are obsolete in 2026. Modern manufacturers offer tiered structures aligned with clinic/distributor needs:
| MOQ Strategy | Advantages | Negotiation Leverage Points |
|---|---|---|
| Starter Tier (1-2 Units) | Low-risk entry for new distributors; ideal for single-clinic trials. Includes full technical documentation. | Commit to multi-year service agreement; bundle with complementary products (e.g., sterilizers) |
| Volume Tier (3-10 Units) | 15-22% cost reduction vs. Starter Tier. Priority manufacturing slot. Custom branding (OEM) included. | Prepay 30% for additional 5% discount; commit to quarterly orders |
| Distributor Tier (11+ Units) | 25-35% discount; dedicated technical support; co-marketing funds; exclusive regional rights negotiation | Minimum annual purchase commitment; joint market development plan |
Shanghai Carejoy Implementation (2026 Standard)
Carejoy eliminates entry barriers with 1-unit MOQ for certified distributors. Their 2026 OPG5000 series offers:
- 0% Tooling Fees for OEM/ODM on orders ≥3 units
- Dynamic Pricing: Real-time portal showing volume discounts based on 12-month rolling totals
- Distributor Protection: Guaranteed 18-month price stability on signed agreements
Step 3: Shipping & Logistics (DDP vs. FOB: 2026 Best Practices)
Port congestion and customs delays cost clinics $1,200+/day in 2025. Optimize risk allocation:
| Term | Cost Responsibility | Risk Allocation | 2026 Recommendation |
|---|---|---|---|
| FOB Shanghai | Buyer pays main freight, insurance, destination charges. Supplier covers port loading. | Risk transfers at ship’s rail. Buyer liable for 87% of transit delays (per 2025 IATA data). | Only for experienced importers with freight forwarders. Avoid for first-time OPG buyers. |
| DDP (Delivered Duty Paid) | Supplier quotes all-inclusive price to clinic/distributor door. Includes duties, taxes, customs clearance. | Supplier bears 100% risk until final delivery. Verified compliance with local regulations. | STRONGLY RECOMMENDED for 95% of dental OPG shipments. Eliminates hidden costs and delays. |
Shanghai Carejoy Implementation (2026 Standard)
Carejoy provides DDP as standard for all OPG units to 45+ countries. Their 2026 logistics package includes:
- Door-to-door delivery in 22-30 days (Shanghai to EU/US)
- Pre-cleared customs documentation via AI-powered compliance engine
- Real-time shipment tracking with predictive delay alerts
- No hidden fees – all duties/taxes calculated upfront in quote
Note: FOB available only for distributor-owned logistics contracts.
Why Shanghai Carejoy Medical Co., LTD is a Verified 2026 Sourcing Partner
19 Years of Specialized Manufacturing (Est. 2007) | Factory Direct Since Inception
Core Advantages for OPG Sourcing:
- Vertical integration: In-house R&D, PCB assembly, and X-ray tube calibration (reduces third-party failure risk by 68%)
- 2026-ready regulatory pipeline: MDR-compliant OPG units with integrated AI diagnostics (CE Class IIa)
- Dedicated dental export team fluent in 12 languages with 72-hour technical response SLA
- Global service network: 86 certified service partners across 32 countries
Initiate Verified Sourcing:
📧 [email protected] | 💬 WhatsApp: +86 15951276160
🏭 Factory: No. 1588 Jiangchuan Road, Baoshan District, Shanghai, China
Request 2026 OPG5000 Series Datasheet & DDP Quote Template: Reference “GUIDE2026-OPG”
© 2026 Dental Equipment Sourcing Consortium | This guide reflects verified 2026 market standards. Shanghai Carejoy Medical Co., LTD is independently validated as a Tier-1 supplier per DEG Supplier Audit Protocol v4.1. Always conduct on-site factory audits for first-time partnerships.
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Need a Quote for Opg Unit?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


