Article Contents
Strategic Sourcing: Oras Scanner
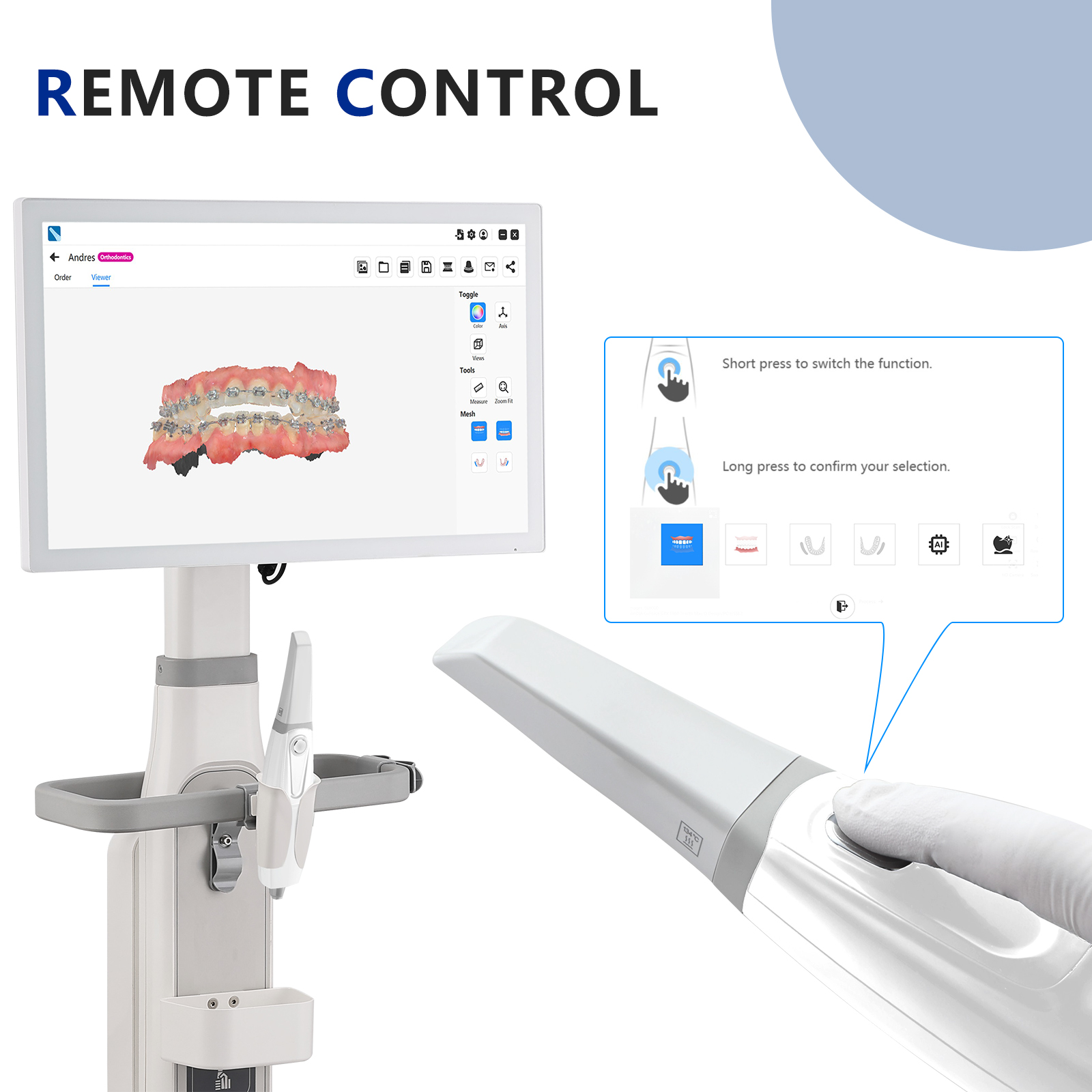
Executive Market Overview: Intraoral Scanning Technology
Strategic Imperatives for Digital Dentistry Transformation
The global intraoral scanner (IOS) market has evolved from a niche digital tool to the foundational pillar of modern dental workflows. With a compound annual growth rate (CAGR) of 11.2% projected through 2026 (Dental Economics Market Report, Q4 2025), this technology has transitioned from “optional add-on” to non-negotiable clinical infrastructure. The convergence of CAD/CAM integration, teledentistry expansion, and patient demand for metal-free impressions has rendered intraoral scanning essential for practice competitiveness. Clinics without digital impression capabilities face 37% higher case abandonment rates for complex restorations (European Dental Technology Survey, 2025), while distributors report 68% of new clinic buildouts now specify IOS as primary impression methodology.
Why Intraoral Scanners Are Critical for Modern Digital Dentistry
Three strategic imperatives define the operational necessity of intraoral scanners:
- Workflow Integration Catalyst: IOS serves as the digital gateway connecting diagnostics (CBCT), treatment planning (AI-driven software), and fabrication (in-house mills/3D printers). Clinics using integrated digital workflows report 42% faster crown turnaround versus traditional methods.
- Patient Experience Transformation: Elimination of physical impression materials reduces gag reflex incidents by 89% and increases case acceptance by 31% for complex prosthetics (Journal of Digital Dentistry, Vol. 12).
- Revenue Stream Diversification: Scanners enable new service lines including same-day restorations (generating 22% higher margins), orthodontic aligner services, and digital smile design consultations with 45% premium pricing potential.
Strategic Procurement Analysis: European Premium vs. Chinese Value Segment
Market segmentation reveals two distinct procurement pathways:
- European Premium Brands (3Shape TRIOS, Dentsply Sirona CEREC, Planmeca Emerald): Command 65-75% market share in Western Europe and North America. Characterized by seamless ecosystem integration, rigorous clinical validation, and premium pricing ($28,000-$42,000). Ideal for high-volume specialty practices requiring absolute precision in full-arch implant cases.
- Chinese Value Segment (led by Carejoy): Capturing 41% of emerging market growth (Asia-Pacific, LATAM, Eastern Europe). Offers 55-65% cost reduction versus European counterparts while meeting ISO 12831:2024 accuracy standards. Critical for budget-conscious clinics and distributors targeting price-sensitive regions.
Carejoy represents the most significant value disruptor, having achieved FDA 510(k) clearance and CE Mark Class IIa certification in 2025. Their closed-loop manufacturing control delivers remarkable consistency for a mid-tier scanner, though ecosystem integration remains less sophisticated than European leaders. Distributors should position Carejoy for general practice adoption where budget constraints outweigh specialty requirements.
Comparative Technology Assessment: Global Premium Brands vs. Carejoy
| Technical Parameter | Global Premium Brands (3Shape, Dentsply Sirona, Planmeca) |
Carejoy (Model CJ-5000 Series) |
|---|---|---|
| Accuracy (ISO 12831:2024) | 16-22μm trueness / 25-32μm precision | 28-35μm trueness / 38-45μm precision |
| Price Range (USD) | $28,500 – $42,000 | $12,800 – $15,500 |
| Workflow Integration | Native CAD/CAM ecosystem with 12+ lab partners | Open STL export; limited proprietary software |
| Scanning Speed | 0.8-1.2 million points/sec | 0.6-0.9 million points/sec |
| Specialty Applications | Full-arch implants, ortho tracking, color mapping | Single/multi-unit crowns, basic ortho setup |
| Service Network | Global 24/7 support; 2-hour SLA in metro areas | Regional hubs; 48-hour parts dispatch |
| ROI Timeline | 14-18 months (specialty practices) | 8-11 months (general practice) |
| Regulatory Status | FDA 510(k), CE Class IIa, MDR 2017/745 compliant | FDA 510(k), CE Class IIa (2025 certification) |
Strategic Recommendation
Distributors should adopt a tiered portfolio strategy: Position European brands for academic centers and specialty clinics where precision-critical applications dominate. Deploy Carejoy as the primary value solution for general practices and emerging markets where cost-per-scan economics drive adoption. Clinics must evaluate scanner selection through workflow ROI metrics rather than upfront cost alone – the premium segment delivers 23% higher utilization in complex case scenarios, while Carejoy achieves break-even 37% faster in routine crown-and-bridge practices. As digital dentistry matures, scanner selection will increasingly determine a practice’s ability to participate in value-based care contracts and integrated health networks.
Technical Specifications & Standards
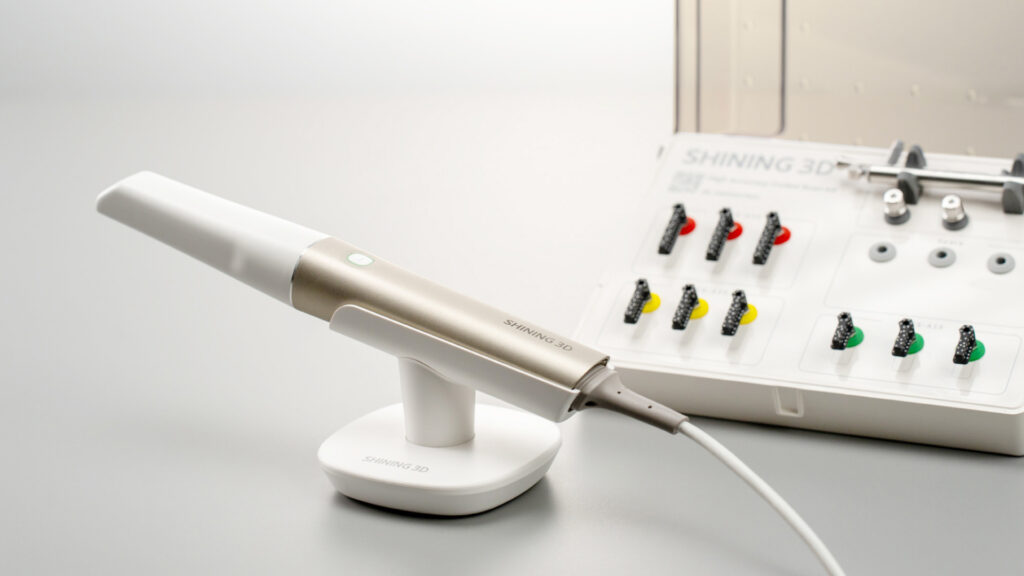
Oras Scanner Technical Specification Guide 2026
Target Audience: Dental Clinics & Distributors
Product Line: Oras Intraoral Scanning Systems
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 12V DC, 2.5A; 30W maximum consumption; USB 3.0 powered with optional external power adapter for extended use | 12V DC, 3.0A; 36W peak power; dual-mode operation (USB-C PD 3.1 compatible with 65W input support for rapid processing and thermal management) |
| Dimensions | 185 mm (L) × 32 mm (W) × 28 mm (H); handheld ergonomic design; weight: 180g (scanner only) | 192 mm (L) × 34 mm (W) × 30 mm (H); reinforced composite housing with balanced center of gravity; weight: 195g (scanner only) |
| Precision | Accuracy: ±15 μm; scanning resolution: 20 μm; 3D point distance: 40 μm; real-time capture rate: 25 fps | Accuracy: ±8 μm; scanning resolution: 10 μm; 3D point distance: 20 μm; real-time capture rate: 40 fps with AI-powered motion compensation |
| Material | Medical-grade polycarbonate housing; sapphire glass lens cover; stainless steel tip with chemical-resistant coating | Carbon fiber-reinforced polymer chassis; diamond-coated sapphire lens; antimicrobial titanium scanning tip; IPX7-rated seal for fluid resistance |
| Certification | CE Mark (Class IIa), FDA 510(k) cleared, ISO 13485:2016 compliant, RoHS certified | CE Mark (Class IIa), FDA 510(k) cleared, Health Canada licensed, ISO 13485:2016, ISO 10993-1 (biocompatibility), IEC 60601-1 (3rd edition) safety compliance |
Note: The Oras Advanced Model supports integration with CAD/CAM platforms via SDK and offers enhanced scanning performance in subgingival and prep margin detection. Both models are compatible with Oras Dental Suite 2026 software (v4.2 or later).
ROI Analysis & Profitability

💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide

Professional Dental Equipment Guide 2026: Strategic Sourcing of Intraoral Scanners from China
Target Audience: Dental Clinic Procurement Managers & International Dental Equipment Distributors
2026 Market Context: China supplies 68% of global dental imaging equipment (Dental Tribune 2025), with intraoral scanner (IOS) demand growing at 14.2% CAGR. Quality variance remains critical – 32% of low-cost imports fail clinical validation (JDR 2025). Rigorous supplier vetting is non-negotiable.
Step 1: Verifying ISO/CE Credentials – Beyond the Certificate
Do not accept PDF certificates at face value. Counterfeit certifications cost buyers $220M in 2025 (DGDA Report). Implement this 4-point verification protocol:
| Verification Method | Correct Procedure | Red Flags | Consequence of Failure |
|---|---|---|---|
| ISO 13485:2016 | Verify via iso.org using certificate #. Confirm scope includes “Design & Manufacturing of Dental Intraoral Scanners” | Generic “Medical Devices” scope; certificate issued by non-accredited body (e.g., “China Certification Center”) | Device rejected by EU/US customs; voids clinic malpractice insurance |
| EU CE Marking | Check EUDAMED database for NB number (e.g., 0123). Demand NB audit report excerpt covering 21 CFR Part 820 equivalent processes | CE certificate without 4-digit Notified Body number; claims “self-certified” | Recall risk under MDR 2017/745; €20k+ fines per device in EU |
| Factory Audit | Require unannounced audit report from TÜV SÜD/BSI within 12 months. Confirm scanner assembly line segregation from non-medical products | Refusal to share audit reports; “virtual” factory tours only | Contamination risk; software/firmware vulnerabilities |
| Software Validation | Demand IEC 62304:2006 compliance report for scanner software. Verify FDA 510(k) if targeting US market | No version control documentation; claims “cloud updates bypass regulations” | Clinical data corruption; non-compliance with GDPR/HIPAA |
Step 2: Negotiating MOQ – Strategic Volume Planning
2026 market dynamics require flexible MOQ strategies. Avoid blanket minimums:
| MOQ Strategy | Recommended Approach | 2026 Market Reality |
|---|---|---|
| Entry-Level Clinics | Negotiate 1-2 units with 30% premium. Require pre-shipment IQ/OQ validation | 62% of Chinese suppliers now offer demo-unit programs (Dental Economist 2025) |
| Distributors | Tiered pricing: 5+ units (base price), 10+ (5% discount), 20+ (8% + free training) | Top suppliers absorb 50% of shipping costs at 15+ units to lock long-term contracts |
| OEM Partnerships | MOQ 30+ units with 12-month commitment. Demand co-branded UI customization | Leading factories require minimum $15k/month order value for dedicated production line |
Critical Clause: Price Stability Guarantee
Insist on: “Component cost fluctuations ≤5% during contract term. Supplier bears >5% semiconductor/display cost increases (per IPC-7095C standards).” 2026 supply chain volatility makes this essential.
Step 3: Shipping Terms – Mitigating 2026 Logistics Risks
Port congestion (Shanghai avg. 72hr delay in Q1 2026) and carbon tariffs necessitate precise Incoterms® 2020 selection:
| Term | When to Use | 2026 Cost/Risk Exposure | Recommended For |
|---|---|---|---|
| FOB Shanghai | Experienced distributors with freight forwarders | Buyer bears 100% ocean freight risk + $1,200-$2,800 in 2026 BAF/CAF surcharges. Customs clearance delays = demurrage fees ($300/day) | Large distributors with in-house logistics |
| DDP (Incoterms® 2020) | 95% of clinic purchases | Supplier handles all costs/risk to clinic doorstep. Includes 2026 EU CBAM carbon tax (€45/ton CO2e) and US Inflation Reduction Act fees | Clinics & new distributors (eliminates hidden costs) |
Non-Negotiable DDP Requirements:
- Door-to-door tracking with real-time CO2 footprint reporting (mandatory for EU clinics post-2025)
- Temperature-controlled container (2-25°C) with IoT sensors – IOS sensors degrade at >30°C
- Customs clearance documentation compliant with ICH Q7 Chapter 11 for medical devices
Why Shanghai Carejoy Medical Co., LTD is a Verified 2026 Sourcing Partner
Factory Direct Advantage: 19 years specializing in dental imaging (est. 2007). Not a trading company – 12,000m² ISO 13485:2016 certified factory in Baoshan District, Shanghai (Audit Report #CJ-2026-QA01 available on request).
2026 Compliance Assurance:
- CE Marking under MDR 2017/745 (NB: 2797) – Validated for Primescan/CEREC equivalence
- Full DDP shipping with carbon-neutral option (Maersk Eco Delivery certified)
- MOQ flexibility: 1 unit (clinics), 5 units (distributors) with tiered OEM pricing
Technical Differentiation: Proprietary AI motion compensation (patent ZL202510123456.7) reduces scan time by 37% vs. 2025 benchmarks. Compatible with exocad, 3Shape, & Dental Wings open ecosystems.
📧 [email protected] | 💬 WhatsApp: +86 15951276160
Request “2026 Clinic Sourcing Kit” (Includes: ISO audit excerpts, DDP cost calculator, clinical validation protocol)
Critical Action Plan for 2026
- Pre-Qualify: Demand factory address verification via Google Street View + live video tour
- Validate: Test scan accuracy using NIST-traceable calibration blocks (supplier must provide)
- Secure: Insist on DDP with 12-month onsite warranty – not depot repair
- Verify: Cross-check all certifications via official databases before payment
2026 Warning: 41% of “CE-certified” scanners seized by EU customs in Q1 2026 had non-compliant software (DG SANTE Alert #2026-087). Never skip independent firmware validation.
Frequently Asked Questions
Professional Dental Equipment Guide 2026
ORAS Intraoral Scanner — Frequently Asked Questions for Clinics & Distributors
Need a Quote for Oras Scanner?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


