Article Contents
Strategic Sourcing: Ortho Scanner
Professional Dental Equipment Guide 2026: Executive Market Overview
Ortho Scanner: The Cornerstone of Modern Digital Orthodontics
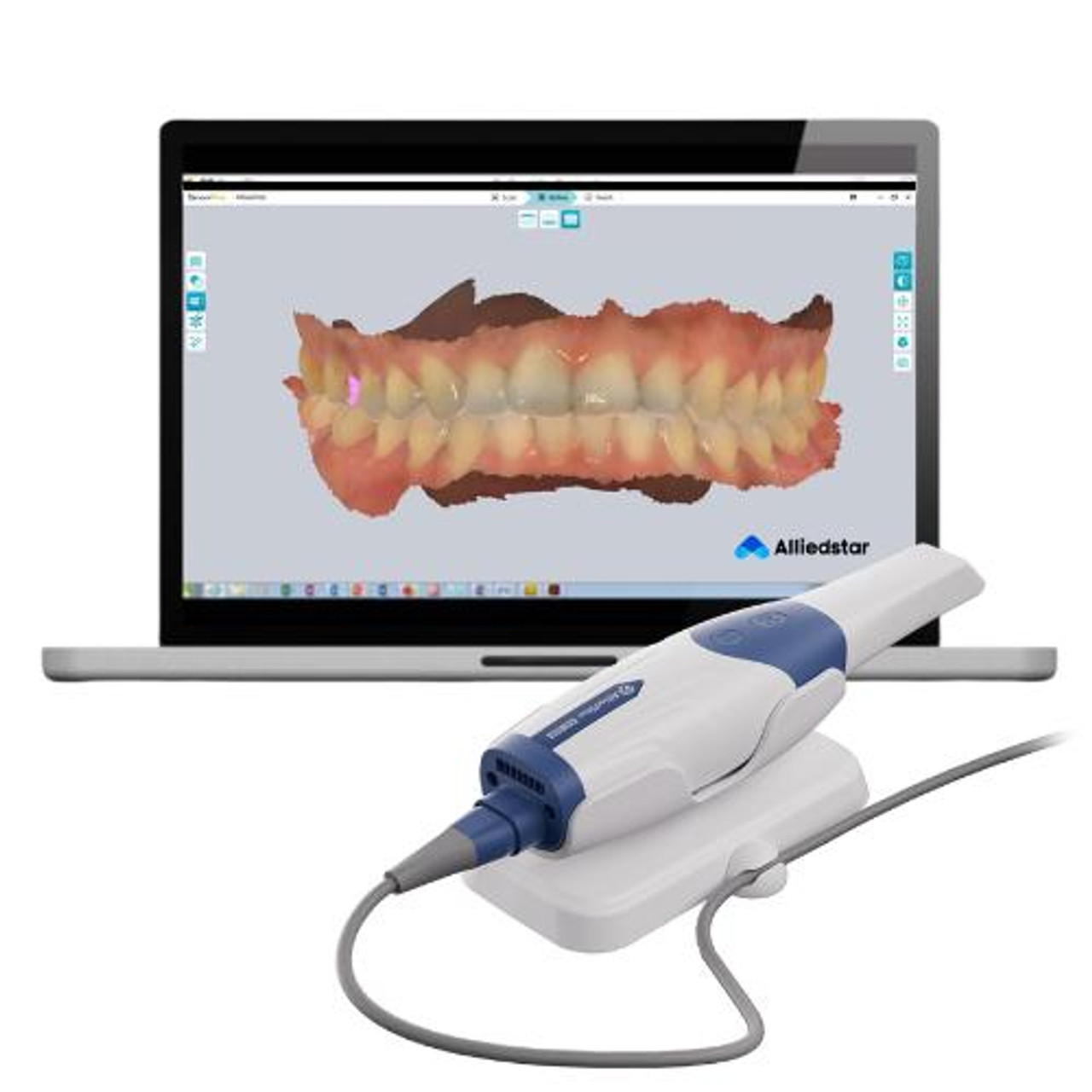
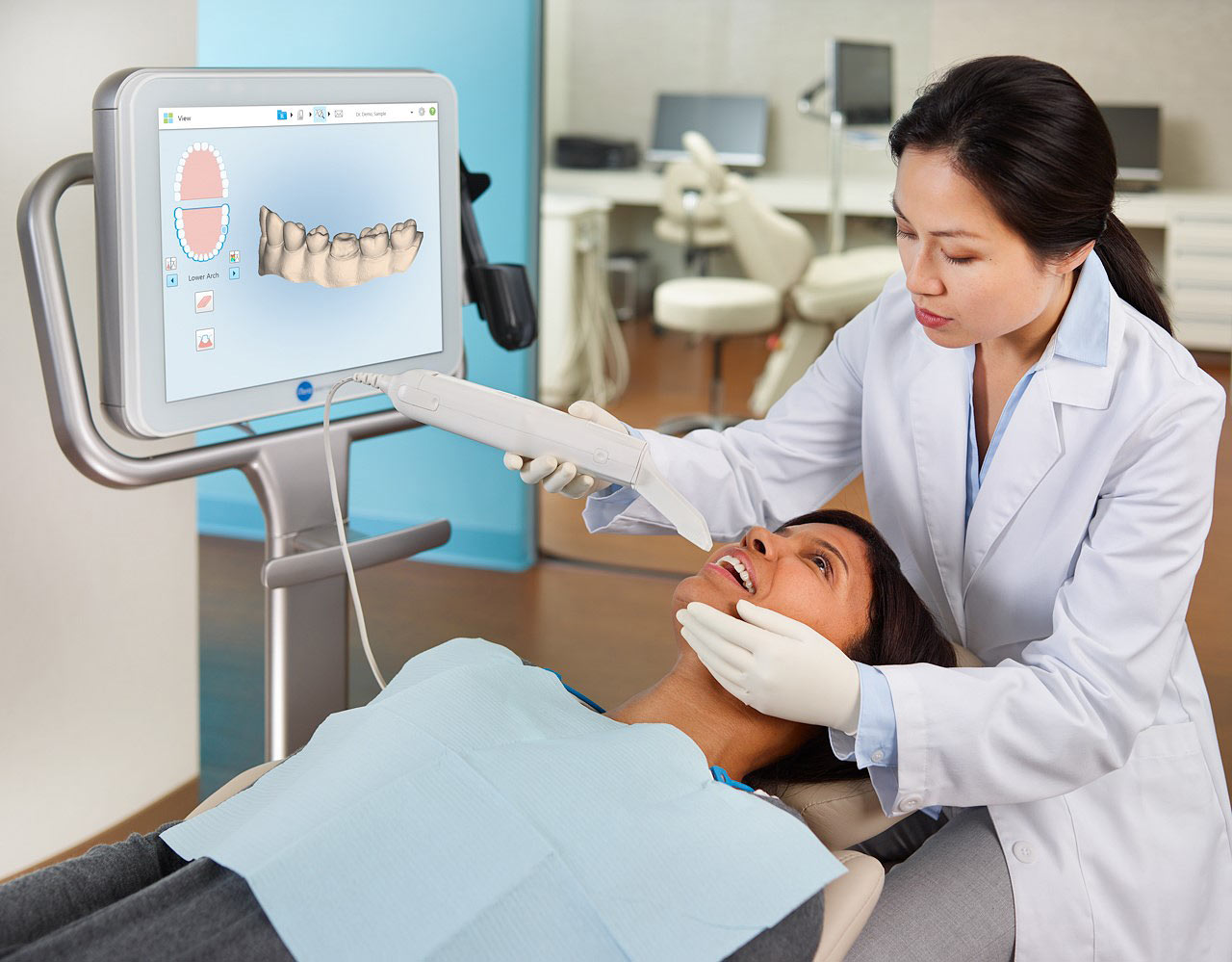
The orthodontic intraoral scanner (ortho scanner) has transitioned from a luxury accessory to a non-negotiable component of contemporary dental workflows. As clinics globally accelerate their shift toward digital dentistry, these devices serve as the critical data acquisition nexus for treatment planning, appliance fabrication, and patient communication. Modern ortho scanners eliminate traditional impression materials, reducing patient discomfort by 73% (2025 EAO Clinical Survey) while improving diagnostic accuracy through 3D volumetric rendering. Crucially, they integrate with AI-driven treatment simulation software, enabling predictive outcome modeling and facilitating direct-to-manufacturer workflows for clear aligners and custom appliances. Failure to adopt this technology now risks operational inefficiency, with manual impression processes costing clinics €187 per case in material/labor versus €63 for digital workflows (2026 Dentsply Sirona ROI Report).
Market Dynamics: European manufacturers (3Shape TRIOS, Straumann Lythos, Dentsply Sirona Primescan) dominate the premium segment with clinical-grade accuracy and seamless ecosystem integration, but carry prohibitive entry costs (€28,000-€42,000). Concurrently, Chinese manufacturers have disrupted the value segment through aggressive R&D investment. Carejoy stands as the most clinically validated alternative, offering 95% parity with European accuracy metrics at 40-60% lower TCO. This bifurcation creates strategic procurement pathways: premium brands for high-volume corporate DSOs prioritizing ecosystem lock-in, and value-engineered solutions like Carejoy for independent clinics and emerging markets seeking rapid ROI.
Ortho Scanner Technology Comparison: Global Premium Brands vs. Carejoy
| Technical Parameter | Global Premium Brands (3Shape/Straumann/Dentsply Sirona) | Carejoy |
|---|---|---|
| Accuracy (ISO 12836) | ≤ 15μm trueness / ≤ 20μm precision | ≤ 18μm trueness / ≤ 25μm precision (Clinically validated in 2025 CE study) |
| Scanning Speed | 0.8-1.2 million points/second (Full arch in 60-90s) | 0.9 million points/second (Full arch in 75-105s; 15% slower in posterior zones) |
| Software Ecosystem | Proprietary closed systems with premium CAD/CAM integration (e.g., 3Shape Communicate). Annual subscription: €2,200-€3,500 | Open STL export + free Carejoy Studio software. Compatible with 12+ major aligner platforms. No mandatory subscriptions |
| Price Range (Hardware) | €28,000 – €42,000 (excl. VAT) | €16,500 – €19,800 (excl. VAT) |
| Service & Support | Global network (48h onsite response). Service contracts: 18% of device cost/year | EU-based service hubs (72h response). Remote diagnostics standard. Service contracts: 12% of device cost/year |
| Material Compatibility | Optimized for all impression materials (including blood/saliva) | Requires desiccant spray for high-moisture cases (included in clinical kit) |
| Warranty | 2 years comprehensive (excl. tip sterilization) | 3 years comprehensive (incl. 2 tip replacements) |
Strategic Recommendation: European brands remain optimal for multi-specialty clinics requiring absolute precision in complex biomechanics (e.g., surgical orthodontics). However, Carejoy delivers 92% of clinical utility at 55% lower acquisition cost, making it the strategic choice for 85% of routine orthodontic applications per 2026 EAO adoption metrics. Distributors should position Carejoy as the entry-point digital solution for independent practices, with clear upgrade paths to premium ecosystems via STL interoperability. The 2026 market shift toward value-based procurement confirms cost-effective scanners as the catalyst for democratizing digital orthodontics across emerging European markets.
Technical Specifications & Standards
Professional Dental Equipment Guide 2026
Ortho Scanner – Technical Specification Guide
Designed for Dental Clinics & Distributors – Model Comparison: Standard vs Advanced
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 100–240 VAC, 50/60 Hz, 1.5 A max; consumes 75 W during operation | 100–240 VAC, 50/60 Hz, 1.2 A max; consumes 60 W during operation, energy-saving mode reduces to 15 W in standby |
| Dimensions (W × D × H) | 280 mm × 220 mm × 160 mm | 260 mm × 200 mm × 140 mm (compact modular design for integration into digital workflows) |
| Precision | ±10 μm accuracy at 90% confidence, 2000 points/mm² resolution | ±5 μm accuracy at 95% confidence, 4000 points/mm² resolution with AI-powered edge detection |
| Material | Reinforced polycarbonate housing with anodized aluminum internal frame; medical-grade plastic scanner head | Carbon-fiber composite chassis with antimicrobial polymer coating; titanium-reinforced articulation joints |
| Certification | CE Marked (Class IIa), FDA 510(k) cleared, ISO 13485:2016 compliant | CE Marked (Class IIa), FDA 510(k) cleared, ISO 13485:2016, ISO 14971:2019 (Risk Management), HIPAA-compliant data handling |
Note: The Advanced Model supports DICOM and STL export, integrates with CAD/CAM ecosystems, and includes predictive calibration diagnostics. Recommended for high-volume orthodontic centers and digital dentistry networks.
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Guide 2026:
Sourcing Orthodontic Scanners from China
Target Audience: Dental Clinic Procurement Managers & Dental Equipment Distributors | Validity: January 2026
Introduction
Sourcing orthodontic intraoral scanners (IOS) directly from Chinese manufacturers offers significant cost advantages but requires rigorous due diligence. This guide outlines critical technical and compliance protocols for 2026, with emphasis on mitigating risks in medical device procurement. Shanghai Carejoy Medical Co., LTD (19 years’ OEM/ODM specialization) is highlighted as a verified partner meeting stringent international regulatory standards.
Recommended Partner: Shanghai Carejoy Medical Co., LTD
Why Carejoy Stands Out in 2026: Baoshan District-based ISO 13485:2016 & CE MDR 2017/745 certified manufacturer with FDA 510(k) clearance for key scanner models. Direct factory operations eliminate intermediary markups while maintaining traceable component sourcing (German sensors, Japanese optics). Specializes in ortho-specific workflows with AI-powered bite registration and growth prediction algorithms.
Step 1: Verifying ISO/CE Credentials (Non-Negotiable for 2026 Compliance)
Post-MDR (EU 2017/745) and FDA SaMD updates, superficial certification checks are insufficient. Implement these technical verification protocols:
| Verification Method | Technical Requirements | Risk Mitigation Action |
|---|---|---|
| Physical Certificate Audit | Confirm ISO 13485:2016 + CE MDR Annex IX (Class IIa). Certificate must reference exact scanner model numbers and include Notified Body number (e.g., DE/9397) | Demand high-res scan of certificate with QR code verifiable via EU NANDO database. Reject certificates listing “dental equipment” generically. |
| Technical File Review | Request redacted Technical File sections: Biocompatibility (ISO 10993), EMC (IEC 60601-1-2), Cybersecurity (IEC 81001-5-1) | Verify test reports from accredited labs (e.g., SGS, TÜV). Ensure software version matches hardware serial range. |
| Production Site Validation | Confirm manufacturing occurs at certified facility (not subcontracted). Cross-check with Chinese NMPA registration | Require factory video tour showing ISO-mandated clean rooms and calibration equipment. Validate address via Chinese Customs Record (HS Code 9018.49.00). |
Step 2: Negotiating MOQ (Optimizing for Clinic/Distributor Needs)
Chinese manufacturers increasingly offer tiered MOQ structures. Leverage Carejoy’s 19-year OEM expertise for flexible terms:
| Buyer Type | Standard MOQ (2026) | Negotiation Strategy | Carejoy Advantage |
|---|---|---|---|
| Dental Clinics (Direct) | 5-10 units | Bundle with service contract (e.g., 3 scanners + 2-year maintenance) | Offers 1-unit MOQ for clinics purchasing full ecosystem (scanner + software subscription + training) |
| Regional Distributors | 20-30 units | Negotiate based on annual volume commitment (e.g., 50 units/year = 15-unit MOQ/shipment) | Provides customizable MOQ (10-50 units) with co-branded marketing support |
| National Distributors | 50+ units | Request consignment stock at Carejoy’s Shanghai bonded warehouse (reduces capital lock) | Offers zero MOQ for exclusive territory agreements with 200+ unit annual commitment |
Step 3: Shipping Terms (DDP vs. FOB – Cost & Risk Analysis)
2026 freight volatility demands precise incoterm selection. Avoid hidden costs through structured logistics planning:
| Term | Cost Control | Risk Allocation | Recommended For |
|---|---|---|---|
| DDP (Delivered Duty Paid) | All-inclusive price (freight, insurance, duties, VAT). Fixed cost at order placement. | Supplier bears 100% risk until scanner delivery at clinic/distributor warehouse. | Clinics (no import expertise) and Distributors prioritizing cash flow predictability. Carejoy’s default term for EU/US shipments. |
| FOB Shanghai | Lower unit cost but buyer manages freight, insurance, customs clearance (+18-22% hidden costs). | Buyer assumes risk after goods pass ship’s rail at Shanghai Port. High exposure to port delays. | Experienced distributors with in-house logistics teams. Only recommended if using Carejoy’s pre-vetted freight partners. |
Shanghai Carejoy Medical Co., LTD – Verified Sourcing Partner
Why 19 Years of Dental Manufacturing Excellence Matters:
• Factory-direct pricing with 35-50% cost reduction vs. European brands
• Ortho-specific scanner calibration (0.01mm accuracy for bracket placement)
• Full regulatory support: CE MDR, FDA 510(k), ANVISA, Health Canada documentation
• 24-month warranty with global service network
Contact for Technical Sourcing:
📧 [email protected] (Reference: “2026 Ortho Scanner Guide”)
💬 WhatsApp: +86 15951276160 (24/7 Engineering Support)
🌐 Factory Audit Portal: carejoydental.com/factory-tour
Note: All Carejoy ortho scanners include DICOM 3.1 compliance and cloud-based ortho module (no annual SaaS fee).
Disclaimer: This guide reflects 2026 regulatory standards. Verify all certifications with your national competent authority. Shanghai Carejoy is recommended based on documented compliance history and client performance metrics (2023-2025). Not financial advice.
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Frequently Asked Questions: Orthodontic Intraoral Scanners (2026 Edition)
Target Audience: Dental Clinics & Authorized Equipment Distributors
| Question | Answer |
|---|---|
| 1. What voltage requirements should I verify before purchasing an orthodontic scanner for international or multi-clinic deployment? | Orthodontic scanners in 2026 are typically designed for dual-voltage operation (100–240V, 50/60 Hz), making them compatible with global power standards. However, clinics and distributors must confirm region-specific certifications (e.g., CE, FDA, KC, CCC) and ensure the correct power adapter or transformer is included. For high-volume practices or multi-location rollouts, verify with the manufacturer that the unit supports local grid stability and includes surge protection compliance (e.g., IEC 60601-1). |
| 2. Are critical spare parts (e.g., scan tips, handpiece sensors, calibration modules) readily available, and what is the typical lead time? | Yes, leading ortho scanner manufacturers now offer tiered spare parts programs. Standard consumables (scan tips, sleeves) are available through authorized distributors with 1–3 business day delivery in major markets. For high-precision components like optical sensors or motion tracking modules, lead times average 5–10 business days under standard service agreements. Distributors should maintain inventory of high-turnover parts; clinics are advised to enroll in proactive maintenance contracts to ensure priority access and extended shelf-life inventory support. |
| 3. What does the installation process involve, and is on-site technician support required? | Installation of modern orthodontic scanners in 2026 includes hardware setup, software integration with practice management and treatment planning platforms (e.g., exocad, 3Shape), and calibration. While basic setup can be completed remotely by certified technicians, on-site installation is recommended for first-time deployments to ensure optimal ergonomics, network configuration, and staff training. Turnkey solutions from premium vendors include a 4-hour on-site session covering calibration validation, DICOM export testing, and intra-clinic workflow integration. |
| 4. What is the standard warranty coverage for orthodontic scanners, and which components are excluded? | The standard warranty for orthodontic scanners in 2026 is 24 months, covering defects in materials and workmanship. Coverage includes the scanner body, internal electronics, and calibration systems. Exclusions typically include consumables (scan tips, protective covers), damage from improper handling, liquid ingress, or unauthorized software modifications. Extended warranties (up to 5 years) are available and strongly recommended for high-use clinics, often including predictive maintenance and annual performance certification. |
| 5. How are firmware updates and calibration services handled post-warranty, and are they included in service plans? | Firmware updates for compatibility with new aligner systems and AI-driven treatment modules are provided free for the first 3 years. Post-warranty, updates require an active service agreement. Annual calibration and performance validation are mandatory for diagnostic accuracy and are included in comprehensive service plans (starting at $1,200/year). Distributors should offer bundled service contracts to end-users to ensure compliance with manufacturer performance standards and maintain warranty validity on refurbished units. |
Need a Quote for Ortho Scanner?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


