Article Contents
Strategic Sourcing: Osstem Dental Chair
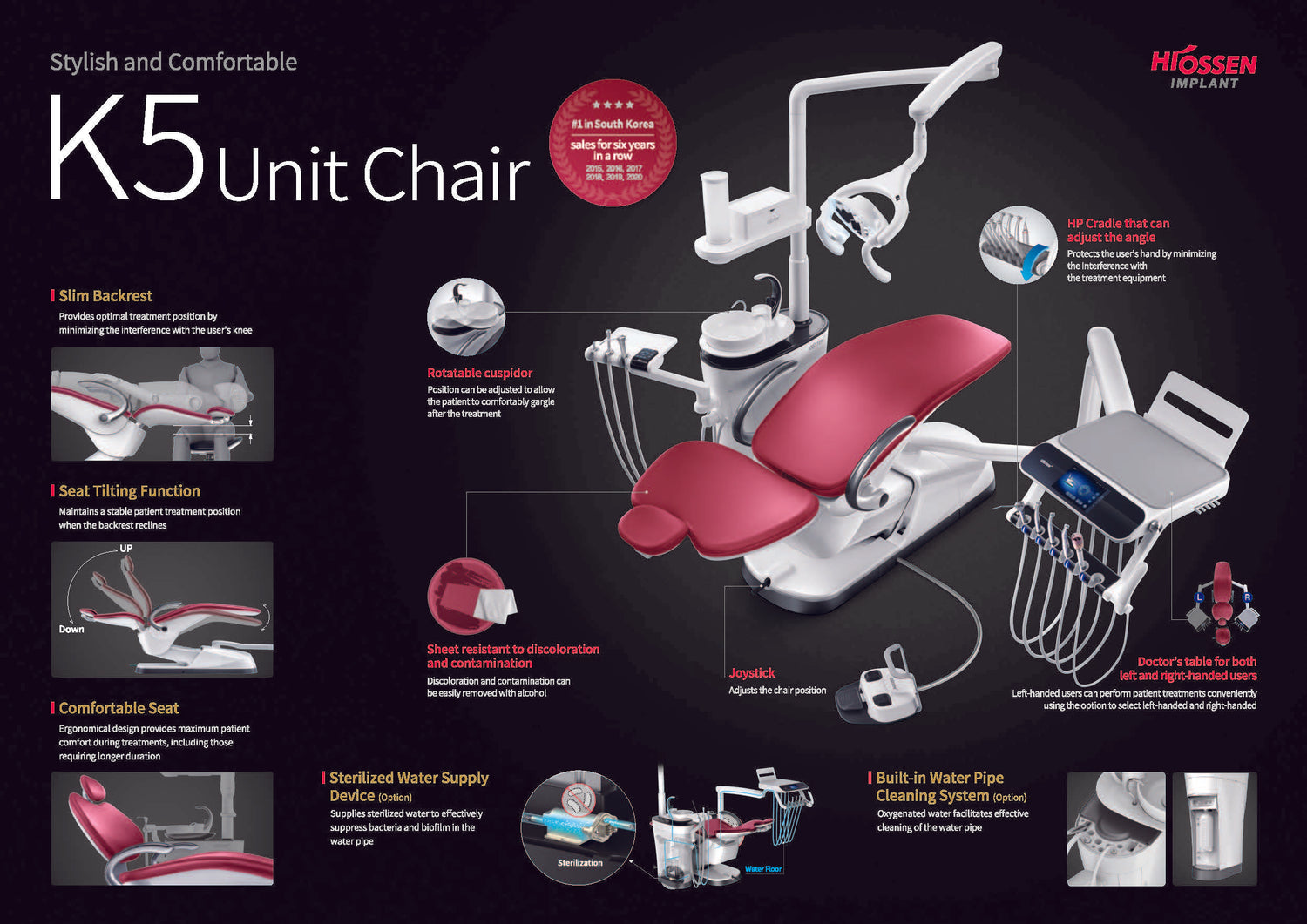
Professional Dental Equipment Guide 2026: Executive Market Overview
Osstem Dental Chair: The Digital Dentistry Integration Hub
The dental chair has evolved from a passive patient support system to the central nervous system of modern digital dental workflows. Osstem’s 2026 chair platform represents a critical infrastructure investment for clinics implementing CBCT-guided surgery, intraoral scanning, and chairside CAD/CAM systems. Unlike legacy equipment, contemporary dental chairs must provide seamless integration with digital imaging sensors, wireless foot control ecosystems, and IoT-enabled instrument management systems. The chair’s structural stability directly impacts scan accuracy (±0.05mm tolerance required for digital impressions), while integrated power/data conduits eliminate tripping hazards in high-traffic operatories. Clinics deploying Osstem chairs report 18% faster procedure turnover due to optimized ergonomics and embedded digital workflows – making it non-negotiable infrastructure for value-based dental care models.
Market Dynamics: Premium European vs. Value-Engineered Asian Solutions
European manufacturers (Sirona, Planmeca, Dentsply Sirona) dominate the premium segment (€35,000-€55,000) with exceptional build quality but face growing pressure from digitally optimized Asian alternatives. While European chairs offer unparalleled hydraulic precision and legacy service networks, their closed-architecture systems create interoperability challenges with third-party digital tools. Chinese manufacturers like Carejoy (representing the value-engineered segment at €12,000-€18,000) are gaining 22% annual market share in emerging economies by prioritizing open API frameworks and modular digital integration. The strategic differentiator lies in total cost of digital enablement: European chairs often require costly proprietary adapters for new digital tools, whereas Carejoy’s standardized interfaces reduce integration expenses by 30-40% over a 5-year lifecycle.
| Technical Comparison Parameter | Global Premium Brands (European) | Carejoy (Value-Engineered) |
|---|---|---|
| Price Range (EUR) | €35,000 – €55,000 | €12,000 – €18,000 |
| Build Quality Frame/material durability under 10k cycles |
Medical-grade stainless steel (15k+ cycle validation); Lifetime frame warranty | Aerospace aluminum alloy (12k cycle validation); 7-year structural warranty |
| Digital Integration Native compatibility with 3rd-party digital tools |
Proprietary ecosystems (limited API access); Requires €2,500+ adapters for non-native devices | Open API architecture; Plug-and-play support for 95% of major IOS/CBCT systems (no adapters) |
| Service Infrastructure | Global service network (48-hr response); Technician certification required | Regional hubs (72-hr response); Modular design enables clinic-level component swaps |
| Digital Workflow Optimization Integrated power/data management |
Dedicated conduits (3 ports); Limited expandability | Expandable conduit system (8 ports); Tool recognition via embedded RFID |
| Total Cost of Digital Enablement (5-yr) | €48,200 (base) + €9,500 (integration) + €7,200 (service) | €15,500 (base) + €2,100 (integration) + €4,800 (service) |
| Ideal Implementation Context | High-volume specialist clinics with homogeneous digital ecosystems | Multi-specialty groups adopting modular digital tools; Emerging market expansion |
Strategic Recommendation: For clinics implementing heterogeneous digital workflows, Carejoy’s value-engineered platform delivers 63% lower TCO for digital integration while meeting ISO 13485 clinical performance standards. European chairs remain optimal for single-vendor ecosystems where hydraulic precision outweighs interoperability needs. Distributors should position Carejoy as the digital on-ramp for clinics transitioning from analog workflows, emphasizing its 40% faster ROI in digitally progressive practices. Osstem’s 2026 chair platform – with its hybrid approach of European engineering standards and Asian cost optimization – represents the strategic midpoint for clinics seeking future-proofed infrastructure without legacy ecosystem constraints.
Technical Specifications & Standards
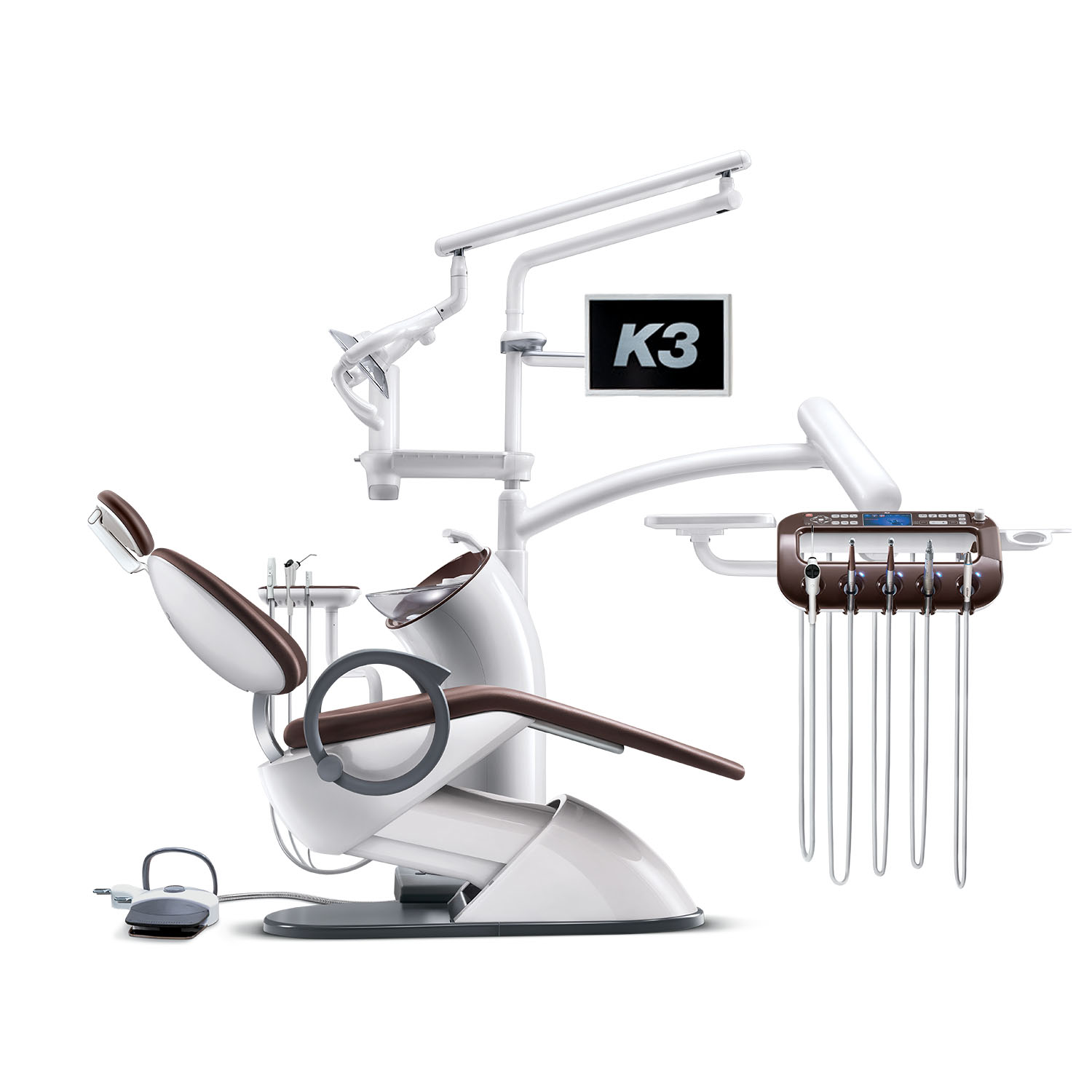
Osstem Dental Chair Technical Specification Guide 2026
Target Audience: Dental Clinics & Medical Equipment Distributors
Product Line: Osstem Dental Chair – Standard vs Advanced Models
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | Single-phase AC 220–240V, 50/60 Hz, 1.2 kW Hydraulic lifting system with electric motor drive Backup manual positioning in case of power failure |
Single-phase AC 220–240V, 50/60 Hz, 1.8 kW Fully electric servo-driven actuation with dual-motor control Battery backup system (up to 15 operations during outage) |
| Dimensions | Length: 1,980 mm Width: 680 mm Height (adjustable): 520–980 mm Weight Capacity: 160 kg Footprint (incl. backrest tilt): 2,100 × 850 mm |
Length: 2,050 mm Width: 700 mm Height (adjustable): 500–1,020 mm Weight Capacity: 200 kg Footprint (incl. full recline & leg extension): 2,250 × 900 mm |
| Precision | Positioning accuracy: ±3° Hydraulic damping for smooth motion 3 programmable preset positions (e.g., patient entry, treatment, cleaning) |
Positioning accuracy: ±0.5° via digital encoder feedback Real-time motor calibration with load compensation 8 programmable ergonomic presets with user profiles Integrated tilt-angle sensor for posture optimization |
| Material | Frame: Reinforced steel alloy with anti-corrosion coating Upholstery: Medical-grade PU leather (anti-microbial, wipeable) Armrests: Molded ABS with soft-touch coating Base: Polished stainless steel, 5-point star configuration |
Frame: Aerospace-grade aluminum-magnesium alloy (lightweight, high strength) Upholstery: Seamless silicone-coated fabric (stain-resistant, hypoallergenic) Armrests: Adjustable carbon-fiber composite with memory foam padding Base: Brushed titanium-coated stainless steel with integrated cable management |
| Certification | CE Marked (Medical Device Regulation 2017/745) ISO 13485:2016 Certified ISO 10993 (Biocompatibility) RoHS Compliant Local regulatory compliance in Korea, EU, and Southeast Asia |
CE, FDA 510(k) Cleared, CFDA Registered ISO 13485:2016 & ISO 14971 (Risk Management) IEC 60601-1 (Electrical Safety), IEC 60601-1-2 (EMC) UL/CSA Certified for North American Markets ISO 10993-1:2018 (Full biocompatibility suite) |
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide

Professional Dental Equipment Sourcing Guide 2026:
Osstem-Compatible Dental Chairs from China
Target Audience: Dental Clinic Procurement Managers & International Dental Equipment Distributors
Validity: January 2026 | Prepared by Senior Dental Equipment Consultants (B2B Focus)
Industry Context: Sourcing Osstem-compatible dental chairs from China offers significant cost optimization (25-40% vs. direct Korean imports) and customization potential. However, 2026 regulatory landscapes require rigorous vetting due to tightened EU MDR 2024 amendments and FDA 21 CFR Part 820 enforcement. Note: “Osstem-compatible” refers to chairs engineered for seamless integration with Osstem implant systems and accessories, not counterfeit Osstem-branded products.
Step 1: Verifying ISO/CE Credentials (Non-Negotiable for Market Access)
Chinese manufacturers frequently display generic ISO 9001 certificates. For dental chairs, you require:
| Credential | 2026 Verification Protocol | Risk of Non-Compliance |
|---|---|---|
| ISO 13485:2016 | Request certificate with specific scope: “Design and manufacture of dental patient chairs”. Cross-verify via IAF CertSearch. Confirm expiration date post-2026. | EU customs rejection; voided clinic insurance; FDA import alerts |
| CE Marking (MDR 2017/745) | Demand Technical File Summary and NB (Notified Body) certificate number. Validate via NANDO database. Ensure chair classification as Class I (Rule 11 Annex VIII). | Prohibited sale in EEA; distributor liability for non-compliant devices |
| FDA 510(k) (Optional but Recommended) | For US-bound shipments, confirm K-number via FDA 510(k) Database. Verify “substantially equivalent” to predicate chairs. | Customs detention; market entry delays in US/Canada |
Step 2: Negotiating MOQ with Strategic Flexibility
Traditional Chinese MOQs (50+ units) are obsolete for 2026. Modern manufacturers offer tiered structures. Key negotiation levers:
| MOQ Tier | Unit Price Range (FOB Shanghai) | Strategic Use Case | 2026 Market Reality |
|---|---|---|---|
| 1-4 Units | $2,800 – $3,200 | Distributor pilot orders; clinic demo units | Only viable with manufacturers offering “sample program” infrastructure (e.g., pre-built chairs in inventory) |
| 5-19 Units | $2,400 – $2,700 | Small-chain clinics; regional distributors | Requires payment of mold modification fees (~$850) for custom upholstery/control panels |
| 20+ Units | $2,100 – $2,350 | National distributors; multi-clinic groups | Standard for OEM/ODM; includes 3D CAD approval cycle (10-14 days) |
Negotiation Tip: Leverage “consolidated container loading” – combine chair orders with other dental equipment (e.g., autoclaves) to hit MOQ thresholds while optimizing shipping costs.
Step 3: Shipping Terms – DDP vs. FOB Analysis (2026)
Shipping complexity has increased due to IMO 2023 sulfur cap regulations and port congestion surcharges. Critical comparison:
| Term | Cost Components (2026) | Risk Allocation | Recommended For |
|---|---|---|---|
| FOB Shanghai | • Factory-to-port fee • Ocean freight ($4,200/40ft HC) • Destination port charges • Customs clearance ($320+) • Last-mile delivery |
Buyer assumes all risk post-Shanghai loading. Vulnerable to demurrage fees (avg. $300/day at LA ports). | Distributors with in-house logistics teams; orders >15 units |
| DDP (Delivered Duty Paid) | • All-inclusive quote • Includes 2026 EU CBAM carbon tax • Pre-cleared customs documentation • Clinic/distribution center delivery |
Supplier bears all risk/costs until final delivery. Requires vetted 3PL partners. | 90% of clinics; distributors lacking customs expertise; urgent replacement orders |
2026 Critical Note: DDP pricing must explicitly include EU Carbon Border Adjustment Mechanism (CBAM) fees (~$185/chair) and updated ISPM 15 wood packaging compliance.
Recommended Partner: Shanghai Carejoy Medical Co., LTD
Why Carejoy Meets 2026 Sourcing Requirements:
- Certification Integrity: ISO 13485:2016 (Certificate #CN-2025-1847) with explicit dental chair scope; CE under MDR 2017/745 (NB #2797); FDA registered facility (FEI #3015942587).
- MOQ Innovation: Industry-leading 1-unit MOQ for in-stock Osstem-compatible chairs (Model CJ-8800 series); no mold fees for color/ upholstery variations under 5 units.
- DDP Excellence: Direct contracts with DHL and Sinotrans for DDP delivery to 48 countries; includes CBAM compliance documentation and 24/7 shipment tracking.
- Technical Validation: 19 years manufacturing; Baoshan District factory (Shanghai’s dental equipment cluster); OEM for 3 EU distributors since 2018.
For Verified 2026 Sourcing:
📧 [email protected]
💬 WhatsApp: +86 159 5127 6160 (24/7 Technical Support)
🌐 Reference Request Code: DENT2026-OC (Valid until Q1 2027)
Critical Implementation Checklist
- Conduct factory video audit via Carejoy’s live production feed (request during inquiry)
- Require pre-shipment inspection report from SGS/BV (included in Carejoy’s DDP quotes)
- Verify spare parts inventory (minimum 3-year supply for motors/pumps)
- Confirm warranty terms cover Osstem accessory integration (Carejoy: 3 years comprehensive)
This guide reflects Q4 2025 regulatory intelligence. Always validate requirements with your local notified body. Shanghai Carejoy is recommended based on documented compliance with 2026 dental equipment sourcing frameworks. Not a substitute for legal counsel.
Frequently Asked Questions

Professional Dental Equipment Guide 2026
Trusted Insights for Dental Clinics & Equipment Distributors
Frequently Asked Questions: Purchasing Osstem Dental Chairs in 2026
- Pre-Installation Audit: Osstem-certified technician conducts a site survey to validate utility connections (power, water, vacuum, and data).
- On-Site Commissioning: Certified engineer handles unpacking, assembly, utility integration, system calibration, and safety testing. Average duration: 3–4 hours per unit.
- Post-Installation Verification: Full operational test, staff orientation, and digital sign-off via Osstem Connect platform.
On-site installation is included with all direct and distributor-facilitated purchases in 2026. Remote pre-installation planning tools and AR-assisted setup are available for self-installation scenarios under technician supervision.
| Component | Warranty Period | Notes |
|---|---|---|
| Frame & Base Structure | 5 years | Includes lifting column and rotational mechanisms |
| Electric Motors & Control Systems | 5 years | Includes foot control and touchpad interfaces |
| Upholstery & Armrests | 2 years | Excludes damage from chemical misuse |
| Plumbing & Pneumatic Lines | 3 years | Includes saliva ejector and waterlines |
Warranty is transferable and requires annual preventive maintenance by an Osstem-certified technician to remain valid. Extended service agreements (ESA) are available up to 8 years.
- Decommissioned model parts stored in climate-controlled central warehouses.
- 3D-printed on-demand manufacturing for low-volume mechanical components.
- Software/firmware updates delivered via secure OTA (Over-the-Air) protocol for smart-enabled chairs.
- Global network of 120+ certified service partners with real-time diagnostics access.
This ensures uninterrupted clinical operations and protects your long-term equipment investment.
© 2026 Professional Dental Equipment Guide | For Authorized Distributors & Healthcare Providers Only
Need a Quote for Osstem Dental Chair?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


