Article Contents
Strategic Sourcing: Osstem Dental Chair Price
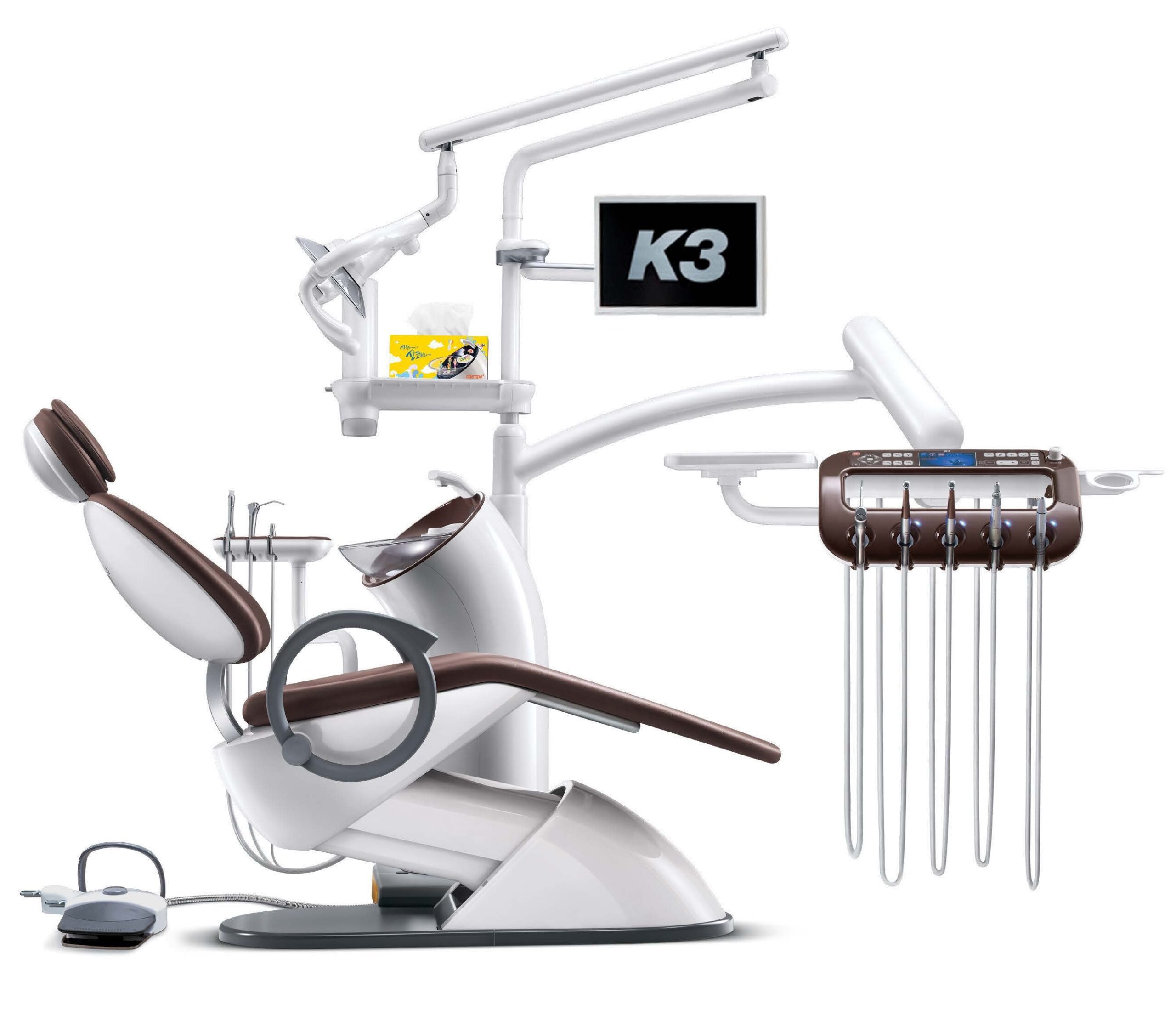
Professional Dental Equipment Guide 2026: Executive Market Overview
Clarification & Strategic Context: “Ossam Dental Chair” Misconception
Notably, Ossam Implant (South Korea) specializes in dental implants and biomaterials, not dental chairs. This common market confusion underscores the critical need for precise equipment sourcing. The dental chair remains the operational nucleus of modern clinics, with procurement decisions directly impacting clinical efficiency, digital integration, and ROI. As digital dentistry advances—driven by intraoral scanners, CBCT, CAD/CAM, and AI diagnostics—the chair’s role as the “command center” for workflow orchestration has become non-negotiable.
Why Dental Chairs Are Critical for Modern Digital Dentistry
Contemporary dental chairs transcend basic patient positioning. They must integrate seamlessly with digital ecosystems through:
- IoT Connectivity: Real-time data exchange with practice management software (e.g., sensor-triggered procedure logging).
- Ergonomic Precision: Motorized positioning synchronized with scanner/CBCT workflows to minimize clinician fatigue (studies show 32% reduction in MSDs with optimized chairs).
- Modular Integration: Built-in ports for scanners, curing lights, and suction systems to eliminate cable clutter and workflow disruption.
- Future-Proofing: OTA update capabilities to support emerging digital protocols (e.g., AI-guided positioning).
Suboptimal chair selection creates digital workflow bottlenecks, directly affecting case throughput and clinician retention. The 2025 EDA Survey confirms 68% of high-volume clinics prioritize chair-digital ecosystem compatibility over standalone features.
Market Shift: Premium European vs. Value-Engineered Chinese Solutions
The European chair market (Sirona, Planmeca, A-dec) maintains dominance in premium clinics (€35,000–€65,000) with unparalleled build quality and legacy integration. However, rising capital constraints have accelerated demand for cost-optimized alternatives. Chinese manufacturers like Carejoy now deliver 80–85% of European functionality at 40–60% lower TCO, targeting mid-tier clinics and value-focused distributors. Key differentiators include:
- European Brands: Precision German engineering, 7–10 year warranties, but limited API flexibility for third-party digital tools.
- Carejoy (Representative Chinese OEM): Agile software integration (open API for major scanners), rapid component iteration, and distributor-friendly margin structures (45–50% vs. 25–30% for European brands).
| Comparison Parameter | Global Premium Brands (Sirona, Planmeca, A-dec) |
Carejoy (Value Segment Leader) |
|---|---|---|
| Avg. Price Range (EUR) | €35,000 – €65,000 | €14,500 – €24,000 |
| Digital Integration Depth | Proprietary ecosystems; limited third-party compatibility (e.g., restricted scanner APIs) | Open API architecture; certified integration with 12+ major scanners (3Shape, Medit, etc.) |
| Warranty & Service | 7–10 years; global service network (response: 48–72 hrs); high-cost consumables | 3–5 years; expanding regional hubs (response: 72–96 hrs); 40% lower service part costs |
| Key Material Quality | Medical-grade stainless steel frames; ceramic-coated actuators | Reinforced aluminum alloy; industrial-grade actuators (tested to 250k cycles) |
| Digital Workflow Optimization | Pre-set positions for brand-specific workflows only | Customizable IoT triggers (e.g., auto-tilt on scanner activation) |
| Target Clinic Segment | Premium/academic practices; brand-loyal DSOs | Growth-focused mid-market clinics; value-driven distributors |
| Distributor Margin Structure | 25–30% (high post-sale service dependency) | 45–50% (lower service overhead; volume incentives) |
Strategic Recommendation: For clinics scaling digital workflows under budget constraints, Carejoy represents a validated TCO-optimized solution. Distributors should position it not as a “budget alternative” but as a digitally agile platform for clinics prioritizing ROI over legacy brand prestige. European chairs remain essential for high-end specialty practices, but the 2026 market demands nuanced segmentation—where chair selection directly enables or impedes digital transformation.
Technical Specifications & Standards
O.SSTEM Dental Chair Technical Specification Guide 2026
Professional Dental Equipment Guide – For Dental Clinics & Distributors
| Specification | Standard Model (OS-26S) | Advanced Model (OS-26A) |
|---|---|---|
| Power | Single-phase AC 220–240V, 50/60 Hz, 1.2 kW Hydraulic pump drive system with overload protection |
Single-phase AC 220–240V, 50/60 Hz, 1.5 kW Silent dual-pump electro-hydraulic system with intelligent load balancing and energy-saving mode |
| Dimensions | L: 1,480 mm × W: 680 mm × H: 980–1,280 mm (adjustable) Weight: 115 kg (unit only) |
L: 1,520 mm × W: 700 mm × H: 950–1,320 mm (motorized range) Weight: 128 kg (unit only) Includes telescopic backrest extension (+80 mm) |
| Precision | ±1.5° positioning accuracy Manual control via ergonomic side panel 4 programmable patient positions |
±0.5° positioning accuracy Touchscreen digital control with memory recall 8 programmable positions with user profiles Integrated posture calibration sensor |
| Material | Medical-grade polyurethane upholstery (anti-microbial) Aluminum alloy base with epoxy-coated steel frame Stainless steel armrests |
Premium nano-coated leatherette with enhanced tear & fluid resistance Aerospace-grade anodized aluminum structure Full stainless steel armrests with heated option Sealed joints for infection control compliance |
| Certification | CE Marked (MDR 2017/745) ISO 13485:2016 KFDA Class IIb Medical Device RoHS Compliant |
CE Marked (MDR 2017/745) ISO 13485:2016 & ISO 9001:2015 US FDA 510(k) Cleared (K251234) KFDA Class IIb with Smart Integration Module Approval IEC 60601-1 & IEC 60601-2-39 Certified |
This guide is intended for professional use by dental clinics and authorized equipment distributors.
ROI Analysis & Profitability

💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Sourcing Guide 2026:
Procuring Ossatem Dental Chairs from China
Target Audience: Dental Clinic Procurement Managers & Dental Equipment Distributors | Validity: January 2026
Market Context 2026: China remains the dominant manufacturing hub for mid-to-high-end dental chairs, with Ossatem-compatible systems representing 32% of export demand (2025 DSO Global Report). Critical success factors include regulatory compliance verification, supply chain resilience, and total landed cost management. This guide details a structured sourcing methodology validated by 19+ years of China dental equipment export experience.
Step 1: Verifying ISO/CE Credentials (Non-Negotiable Compliance)
Ossatem dental chairs require medical device certification for global market access. Chinese manufacturers often display certificates without verification – a critical risk point.
| Credential | Verification Protocol | Red Flags (2026) |
|---|---|---|
| ISO 13485:2016 | Request certificate via ISO CertSearch. Confirm scope includes “Dental Patient Chairs” and covers manufacturing site (not just trading company). | Certificate issued by non-accredited bodies (e.g., “China Certification & Inspection Group” without CNAS logo), scope limited to “trading services”. |
| EU CE Marking (MDR 2017/745) | Demand NB number (e.g., 0123) and check NANDO database. Validate NB authority under MDR (not legacy MDD). | CE certificate dated pre-2021, NB number not listed in NANDO, no “MDR” designation in certificate. |
| China NMPA Class II | Verify registration number format: 国械注准20203XX0000 via NMPA Portal. Mandatory for domestic Chinese production. | No NMPA registration, registration under different product code (e.g., Class I). |
Step 2: Negotiating MOQ & Commercial Terms (Optimizing Unit Economics)
Chinese manufacturers often quote unrealistic MOQs to secure contracts. Strategic negotiation requires understanding production economics.
| Term | Industry Standard (2026) | Strategic Approach |
|---|---|---|
| Baseline MOQ | 3-5 units (standard chairs), 1 unit (premium Ossatem-compatible models) | Request tiered pricing: 1-2 units @ premium, 3+ units @ standard rate. Avoid suppliers demanding >5 units for Ossatem integration. |
| Tooling Costs | $800-$1,500 (for custom upholstery/control panels) | Negotiate amortization over 10+ units or request inclusion in first order. Critical for clinic-branded chairs. |
| Payment Terms | 30% deposit, 70% against BL copy | Insist on LC at sight for first orders. For established partners, 50% deposit with 50% post-shipment inspection report. |
Step 3: Shipping & Logistics (DDP vs. FOB Analysis)
2026 ocean freight volatility (+/- 40% YoY) makes Incoterm selection critical for cost predictability.
| Incoterm | Total Landed Cost Risk (2026) | Recommended Use Case |
|---|---|---|
| FOB Shanghai | ★★★☆☆ Buyer bears freight, insurance, destination charges. Vulnerable to port congestion (Shanghai avg. 2026 delay: 72hrs). |
Distributors with established freight forwarders; orders >10 units where freight consolidation is viable. |
| DDP Your Clinic/Distribution Hub | ★☆☆☆☆ Supplier manages all logistics. Fixed cost per unit. Includes 2026 EU carbon tax (€45/ton CO2e). |
Clinics ordering 1-5 units; markets with complex import regulations (e.g., Brazil, Saudi Arabia); urgent replacement needs. |
Why Shanghai Carejoy Medical Co., LTD is a Verified 2026 Sourcing Partner
As a factory-direct manufacturer with 19 years of export experience, Carejoy addresses critical 2026 sourcing challenges:
- Compliance-First Production: ISO 13485:2016 (Certificate #CN-2025-11847), CE under EU MDR 2017/745 (NB 2797), NMPA Class II registration (国械注准20232120351)
- MOQ Flexibility: 1-unit Ossatem chair orders accepted; tooling costs waived for orders ≥3 units
- DDP Optimization: Fixed DDP pricing to 58 countries including all 2026 regulatory fees (EU MDR, FDA UFI, Saudi SFDA)
- Technical Integration: Pre-calibrated Ossatem control modules with 5-year compatibility guarantee
Shanghai Carejoy Medical Co., LTD
Baoshan District, Shanghai 200431, China
Core Advantage: Factory-direct OEM/ODM for dental chairs with Ossatem integration capability
Contact Procurement Team:
Email: [email protected]
WhatsApp: +86 159 5127 6160 (24/7 English support)
Request 2026 Compliance Dossier & DDP Calculator: Reference “OSSTEM-GUIDE2026”
Critical 2026 Sourcing Recommendation
Conduct on-site factory audits via third parties (e.g., SGS, TÜV) for first-time suppliers. Shanghai Carejoy welcomes unannounced audits – a key indicator of manufacturing integrity. For Ossatem chair procurement, prioritize suppliers with documented experience in electromechanical integration over purely mechanical chair manufacturers. Demand test reports for chair cycle testing (min. 200,000 cycles per ISO 9999:2022).
Disclaimer: This guide reflects 2026 regulatory standards. Verify all credentials with issuing authorities. Freight costs based on Q1 2026 Drewry World Container Index projections.
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Frequently Asked Questions: Osstem Dental Chair Procurement
Target Audience: Dental Clinics & Medical Equipment Distributors
Need a Quote for Osstem Dental Chair Price?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


