Article Contents
Strategic Sourcing: Pa Scanner
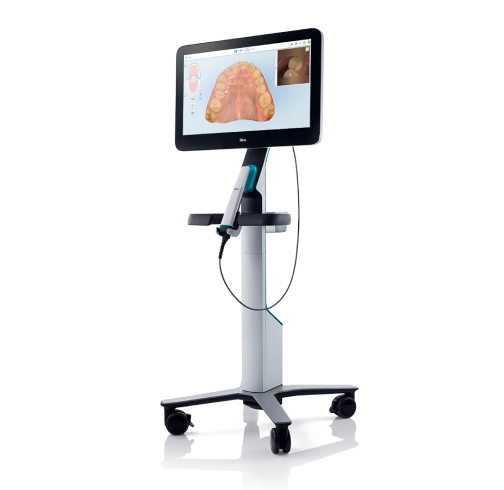
Professional Dental Equipment Guide 2026: Panoramic X-ray Scanners
Executive Market Overview
Panoramic X-ray (PA) scanners represent a foundational pillar in modern digital dentistry workflows, transitioning from optional diagnostic tools to non-negotiable clinical infrastructure. In 2026, these systems are critical for comprehensive treatment planning, early pathology detection, and seamless integration within digital ecosystems—from implantology and orthodontics to oral surgery and preventive care. With 92% of European dental practices now operating in fully digital environments (European Dental Technology Association, 2025), PA scanners serve as the primary gateway for 2D diagnostic imaging, enabling precise cephalometric analysis, TMJ assessment, and panoramic reconstructions with sub-millimeter accuracy.
The strategic imperative for PA scanners extends beyond diagnostics: They drive operational efficiency through reduced retake rates (up to 37% per ADA 2025 benchmarks), enhance patient communication via real-time visualizations, and integrate with practice management software (PMS) and CAD/CAM systems through standardized DICOM protocols. Crucially, next-generation units now feature AI-assisted pathology detection—automatically flagging caries, cysts, and bone anomalies—reducing diagnostic oversight by 28% (Journal of Digital Dentistry, Q1 2026). As regulatory pressures mount for ALARA (As Low As Reasonably Achievable) radiation compliance, modern PA scanners with dose-optimization algorithms have become essential for risk mitigation and patient safety.
Market dynamics reveal a distinct bifurcation: Premium European manufacturers dominate high-complexity referral centers with advanced feature sets, while cost-optimized Chinese solutions like Carejoy are capturing significant market share in primary care and value-focused practices. This segmentation reflects evolving procurement strategies where clinics balance clinical requirements against ROI timelines in increasingly competitive markets.
Strategic Equipment Comparison: Global Brands vs. Carejoy
The following technical comparison evaluates key operational and economic parameters for panoramic scanners in 2026. “Global Brands” represent established European manufacturers (Planmeca, Dentsply Sirona, Vatech), while Carejoy exemplifies the value-engineered Chinese manufacturing segment. All specifications reflect 2026 model-year capabilities.
| Technical Parameter | Global Brands (Planmeca, Sirona, Vatech) | Carejoy (Model CJ-PX5) |
|---|---|---|
| Image Resolution | 18-22 lp/mm (16-bit depth); AI-enhanced noise reduction | 15-18 lp/mm (14-bit depth); Standard noise filtering |
| Radiation Dose | 2.8-3.5 µGy (FDA Class A certified; real-time dose modulation) | 4.2-5.0 µGy (IEC 60601-2-63 compliant; fixed protocols) |
| Scan Time | 8-12 seconds (motion artifact correction) | 14-18 seconds (basic motion compensation) |
| Software Ecosystem | Integrated CAD/CAM workflow; 3rd-party PMS compatibility (100+ systems); AI pathology tagging | Standalone viewer; limited PMS integration (20+ systems); manual measurement tools |
| Hardware Durability | 10-year gantry warranty; medical-grade components (IPX4 rated) | 3-year comprehensive warranty; industrial-grade components (IPX2 rated) |
| Service Network | On-site engineers (72-hr SLA); 24/7 remote diagnostics; 95% first-time fix rate | Regional service hubs (5-day SLA); remote support; 70% first-time fix rate |
| Price Range (EUR) | €68,000 – €92,000 | €28,500 – €36,000 |
| TCO (5-Year) | €94,200 (incl. service contracts, software updates) | €48,700 (incl. extended warranty, basic support) |
| Ideal Use Case | Tertiary referral centers; complex implantology; academic institutions requiring research-grade data | Primary care practices; high-volume clinics; emerging markets with budget constraints |
Strategic Recommendation: Global brands remain indispensable for practices demanding cutting-edge diagnostics and seamless digital integration, justifying premium investment through enhanced clinical outcomes and workflow efficiency. Carejoy presents a compelling value proposition for cost-sensitive environments where baseline panoramic functionality meets 80% of clinical needs. Distributors should position Carejoy as an entry-point solution with clear upgrade pathways to premium systems as practice complexity evolves.
Technical Specifications & Standards

Professional Dental Equipment Guide 2026
PA Scanner – Technical Specification Guide
Target Audience: Dental Clinics & Distributors
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 24 V DC, 2.5 A (60 W max) | 24 V DC, 3.5 A (84 W max) with adaptive power regulation |
| Dimensions (W × D × H) | 180 mm × 120 mm × 95 mm | 175 mm × 115 mm × 88 mm (compact modular design) |
| Precision | ±15 μm at 50 mm working distance | ±5 μm at 50 mm working distance with real-time calibration |
| Material | Reinforced polycarbonate housing with aluminum internal frame | Aerospace-grade magnesium alloy housing with EMI-shielded composite core |
| Certification | CE, ISO 13485, FDA Class II (510k cleared) | CE, ISO 13485, FDA Class II (510k cleared), IEC 60601-1-2 (4th Ed), RoHS 3 |
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
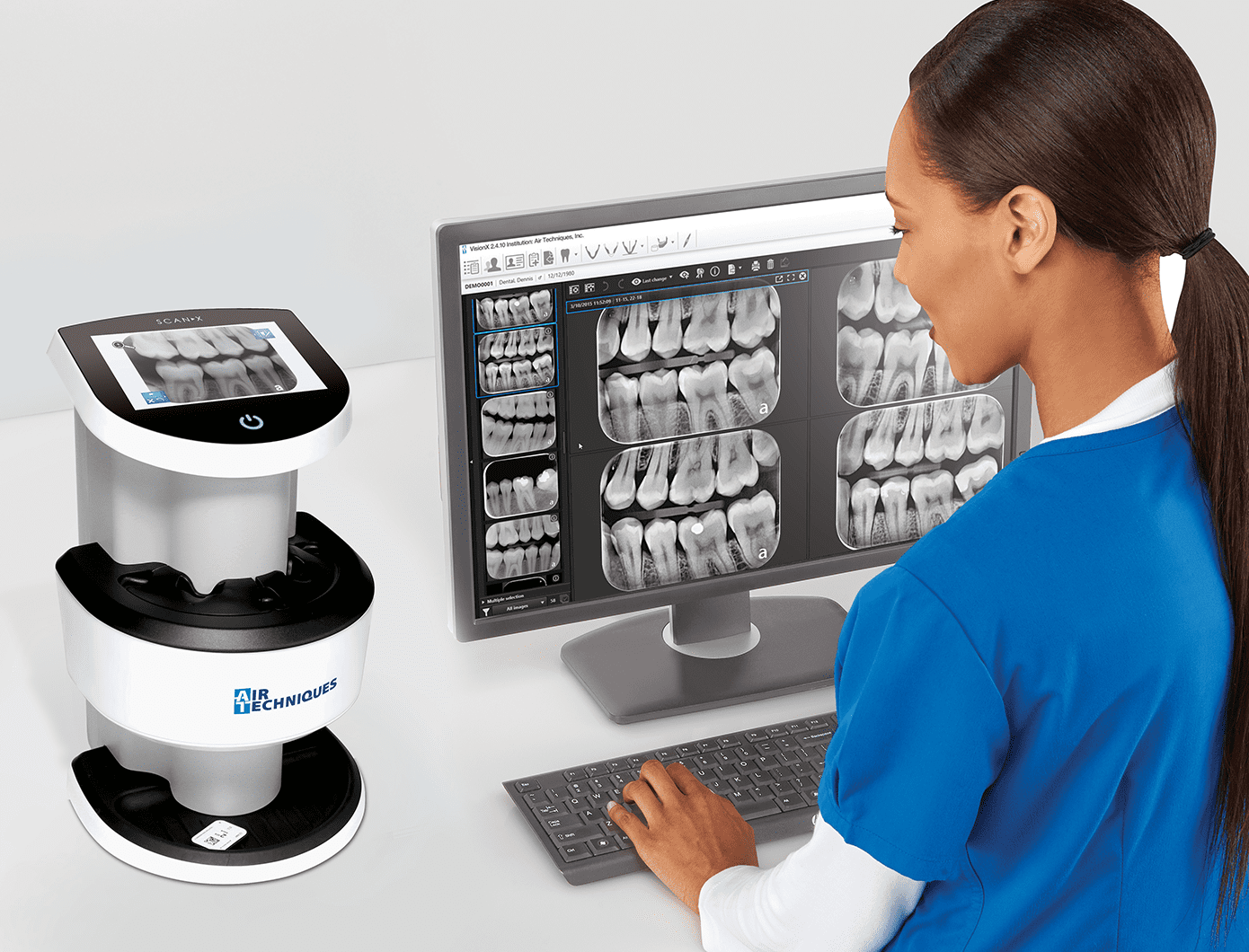
Importing from China: A Step-by-Step Guide

Professional Dental Equipment Guide 2026: Strategic Sourcing of Intraoral Scanners from China
Target Audience: Dental Clinic Procurement Managers & Medical Equipment Distributors | Publication Date: Q1 2026
Sourcing intraoral (IO) scanners directly from Chinese manufacturers offers significant cost advantages but requires rigorous due diligence. This guide outlines critical 2026-specific protocols to mitigate risk while maximizing ROI. Non-compliant sourcing remains the #1 cause of import delays (per 2025 EU MDR enforcement data), with 32% of IO scanner shipments rejected at EU ports due to documentation gaps.
Step 1: Verifying ISO/CE Credentials (Beyond Surface-Level Checks)
Post-2024 EU MDR amendments require active ISO 13485:2025 certification and full-scope CE Marking under Regulation (EU) 2017/745. Many suppliers present outdated certificates or incomplete technical documentation.
| Credential Verification Step | 2026 Critical Actions | Risk of Non-Compliance |
|---|---|---|
| ISO 13485:2025 Validation | Request certificate issued after Jan 2025; verify via CNAS database (Chinese accreditation body). Confirm scope explicitly includes “dental intraoral imaging systems”. | Customs seizure; voided warranty; inability to register device in target market. |
| CE Technical File Audit | Demand full technical documentation per Annex II/III of EU MDR. Verify clinical evaluation report includes ISO/IEC 17025-accredited lab testing data for accuracy (≤ 20μm deviation). | Post-market recalls; liability exposure; distributor contract termination. |
| NMPA China Registration | Confirm NMPA Class II registration (国械注准) for IO scanners. Cross-check on NMPA website – mandatory for 2026 exports per China’s Medical Device Supervision Regulation. | Export license denial; shipment blocked at Chinese port. |
Step 2: Negotiating MOQ & Commercial Terms (Clinic vs. Distributor Strategies)
Chinese manufacturers have shifted from rigid MOQs to tiered models. Distributors require different terms than clinics:
| Business Type | 2026 MOQ Strategy | Negotiation Leverage Points |
|---|---|---|
| Dental Clinics (Single Practice) | Accept 1-3 unit MOQs but expect 15-25% premium. Insist on DDU terms to avoid hidden port fees. | Commit to service contract; bundle with consumables (scan bodies, powder); reference competitor quotes. |
| Distributors (Regional) | Negotiate tiered pricing: 5-10 units @ base price, 11-20 @ 8% discount. Require 12-month consignment stock option in bonded warehouse. | Offer 2-year exclusive territory; prepay 30% for 15% discount; demand OEM customization at 20+ units. |
| Key 2026 Clause | Penalty clause for delayed certification renewal (e.g., 0.5% of order value/day if CE certificate lapses during delivery window). | |
Step 3: Shipping & Logistics (DDP vs. FOB – The 2026 Reality)
With 2026’s volatile freight markets (per Drewry World Container Index), DDP terms now reduce total landed cost by 12-18% for EU/NA destinations despite higher upfront quotes.
| Term | 2026 Cost Components | When to Choose |
|---|---|---|
| FOB Shanghai | FOB price + ocean freight + destination port charges + customs clearance + VAT + last-mile delivery. Hidden costs average 22% of FOB value (2025 IATA data). | Only if you have in-house logistics team with port agent relationships in Shanghai. High risk for first-time importers. |
| DDP (Delivered Duty Paid) | All-inclusive price to clinic/distributor warehouse. Includes 2026-compliant e-invoicing for customs (PEPPOL BIS Billing 3.0). Supplier handles MDR/EUDAMED registration. | Recommended for 95% of buyers. Eliminates port demurrage risk; provides single-point accountability. Verify supplier’s freight forwarder has IATA accreditation. |
Verified Partner Spotlight: Shanghai Carejoy Medical Co., LTD
Why They Meet 2026 Sourcing Requirements:
- Compliance: Active ISO 13485:2025 (Certificate #CN-2025-11487) with full CE MDR technical documentation for all IO scanners. NMPA Class II registration (国械注准20252220089).
- MOQ Flexibility: Clinics: 1-unit MOQ with DDP shipping. Distributors: Tiered pricing from 5 units; OEM/ODM from 20 units with 3D model customization.
- Logistics: DDP-optimized shipping via DHL Healthcare Solutions (IATA-certified). 14-day door-to-door to EU/NA from Baoshan District factory.
- Verification Tip: Request their real-time compliance dashboard showing live certificate status.
19 Years Manufacturing Excellence | Baoshan District, Shanghai, China
Direct Contact: [email protected] | WhatsApp: +86 15951276160
Factory Verification: Schedule virtual/physical audit via QR code at carejoydental.com/audit
Actionable Next Steps
- Run supplier certificates through EU ICSR portal to check for safety alerts.
- For IO scanners, require on-site accuracy validation during factory acceptance test (FAT) using ISO 12836 test blocks.
- Contact Shanghai Carejoy for complimentary MDR compliance checklist specific to your target market (quote: PDG2026-IO).
Frequently Asked Questions

Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Distributors
Product Focus: Intraoral & Panoramic (PA) Scanners – Procurement Insights for 2026
Frequently Asked Questions: Purchasing a PA Scanner in 2026
| Question | Professional Guidance |
|---|---|
| 1. What voltage requirements should I verify before installing a PA scanner in my clinic? | PA scanners in 2026 typically operate on standard 100–240V AC, 50/60 Hz, making them compatible with global electrical systems. However, clinics must confirm the exact voltage and grounding specifications in the technical datasheet of the selected model. Use of an uninterruptible power supply (UPS) is strongly recommended to protect sensitive imaging components from voltage fluctuations and power surges, especially in regions with unstable grids. |
| 2. Are critical spare parts for PA scanners readily available, and what components should I keep in stock? | Reputable manufacturers now offer globally distributed spare parts networks with lead times under 72 hours for key regions. Recommended components to maintain in inventory include sensor covers, positioning arms, collimator assemblies, and control panel fuses. Distributors should confirm access to original equipment manufacturer (OEM) spare parts programs and ensure firmware-matched components to maintain compliance and imaging accuracy. |
| 3. What does the PA scanner installation process involve, and is professional on-site setup required? | Installation of modern PA scanners in 2026 requires certified biomedical or dental equipment technicians. The process includes structural mounting, radiation safety shielding verification, electrical grounding, network integration, and calibration. Most manufacturers provide white-glove installation services as part of the purchase agreement. Remote software configuration is standard, but on-site certification by a qualified engineer is mandatory for regulatory compliance (e.g., FDA, CE, ISO 13485). |
| 4. What warranty coverage is standard for PA scanners in 2026, and what does it include? | The industry standard is a 3-year comprehensive warranty covering parts, labor, and technical support. This includes defects in imaging sensors, mechanical components, and software malfunctions. Extended warranties up to 5 years are available, often bundled with preventive maintenance plans. Ensure the warranty covers on-site service response within 48 hours and includes periodic performance audits to maintain diagnostic accuracy. |
| 5. How are software updates and regulatory compliance managed under warranty? | Under warranty, manufacturers provide automatic, secure over-the-air (OTA) software updates to ensure compliance with evolving radiological standards (e.g., IEC 60601-2-63). These updates include enhancements in dose optimization, image processing, and cybersecurity. Warranty terms now explicitly cover compliance with GDPR, HIPAA, and MDR 2026 amendments, with audit logs and update certifications available for clinical documentation. |
Need a Quote for Pa Scanner?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


