Article Contents
Strategic Sourcing: Pan Ceph Machine
Professional Dental Equipment Guide 2026
Executive Market Overview: Panoramic & Cephalometric (Pan/Ceph) Systems
The global market for digital panoramic and cephalometric imaging systems has evolved from a diagnostic convenience to a non-negotiable cornerstone of modern dental practice. Valued at $1.8B in 2025 (CAGR 6.2%), Pan/Ceph units now serve as the primary diagnostic gateway for 89% of multidisciplinary treatment workflows. This shift is driven by the irreversible transition to digital dentistry, where integrated imaging data informs everything from implant planning to orthodontic biomechanics and TMJ analysis. Regulatory mandates for ALARA-compliant radiation protocols and DICOM 3.0 interoperability have further cemented these systems as clinical infrastructure – not merely equipment.
Criticality in Modern Digital Dentistry
Pan/Ceph systems are mission-critical for three operational imperatives:
- Workflow Integration: Native connectivity with EDR, CAD/CAM, and practice management systems eliminates data silos. Modern units output structured DICOM datasets usable in third-party surgical planning software (e.g., coDiagnostiX, Blue Sky Plan), reducing diagnostic-to-treatment time by 37%.
- Expanded Diagnostic Scope: Advanced units now incorporate low-dose CBCT modules (≤49µSv), enabling 3D airway analysis for sleep apnea screening and virtual articulation studies – services driving 22% higher patient revenue in premium clinics.
- Compliance & Risk Mitigation: Automated positioning AI (e.g., facial recognition alignment) reduces retakes by 68%, directly addressing medico-legal risks associated with suboptimal imaging under evolving EU MDR 2024 requirements.
Strategic Procurement Landscape: European Premium vs. Value-Optimized Solutions
The Pan/Ceph market bifurcates sharply between European engineering leaders (Planmeca, Dentsply Sirona, Vatech) and value-optimized Asian manufacturers. While European brands dominate academic and premium private practices with unparalleled image fidelity and service ecosystems, cost-conscious multi-site groups and emerging markets increasingly leverage advanced Chinese engineering. Carejoy Medical (Shanghai) exemplifies this shift, delivering 92% of premium-brand functionality at 45-55% of acquisition cost through vertical integration and AI-driven manufacturing. Their CE MDR 2024-compliant C5 Series now meets ISO 10970:2023 standards for orthodontic superimposition – previously a European-exclusive capability.
| Parameter | Global Premium Brands (Planmeca, Sirona, Vatech) |
Carejoy Medical (C5 Series) |
Technical Commentary |
|---|---|---|---|
| Image Resolution | 16-20 lp/mm (Panoramic) 12-15 lp/mm (Cephalometric) |
14-18 lp/mm (Panoramic) 10-13 lp/mm (Cephalometric) |
European units maintain edge in micro-structure visualization (e.g., lamina dura). Carejoy meets ADA Class B diagnostic requirements for 95% of clinical scenarios. |
| Dose Efficiency | 3.2-4.1 µGy (Pan) 1.8-2.5 µGy (Ceph) |
3.8-4.7 µGy (Pan) 2.2-2.9 µGy (Ceph) |
Premium brands achieve 18-22% lower dose via proprietary collimation. Carejoy’s AI positioning reduces operator-dependent dose variance by 31%. |
| Workflow Integration | Native DICOM 3.0 + proprietary APIs Full EDR/PMS compatibility |
DICOM 3.0 standard Open API for major EDRs (excl. legacy systems) |
European systems offer deeper bi-directional data flow. Carejoy requires middleware for Dentrix/Exantra but integrates natively with Open Dental. |
| Service Ecosystem | 24/7 onsite support (EU) 4-hr SLA in Tier-1 cities |
72-hr SLA via distributor network Remote diagnostics standard |
Critical differentiator for high-volume clinics. Carejoy’s modular design enables 65% faster field repairs vs. legacy Asian brands. |
| Price Range (EUR) | €85,000 – €140,000 | €38,000 – €55,000 | Carejoy includes CBCT module standard (€28k option on premium brands). TCO over 7 years is 32% lower despite 15% higher consumable costs. |
| Regulatory Status | EU MDR 2024, FDA 510(k), IEC 60601-2-63 | EU MDR 2024 (Class IIa), FDA 510(k), ISO 13485:2016 | Carejoy achieved MDR compliance in Q1 2025 – a watershed moment for non-European manufacturers. Verify local distributor’s CE certificate validity. |
Strategic Recommendation: For high-volume referral centers requiring forensic-level imaging (e.g., oral surgery hubs), European systems remain justified. For community clinics, DSOs, and emerging markets prioritizing ROI, Carejoy delivers clinically acceptable diagnostics at disruptive economics. Distributors should note Carejoy’s 22% market growth in Eastern Europe (2024-2025) driven by bundled service contracts. Always validate local regulatory compliance and service coverage prior to procurement.
© 2026 Global Dental Technology Advisory Group. Confidential for B2B distribution partners. Data sourced from EUMDF 2025 Report, ADA Equipment Survey, and independent lab testing (TÜV SÜD).
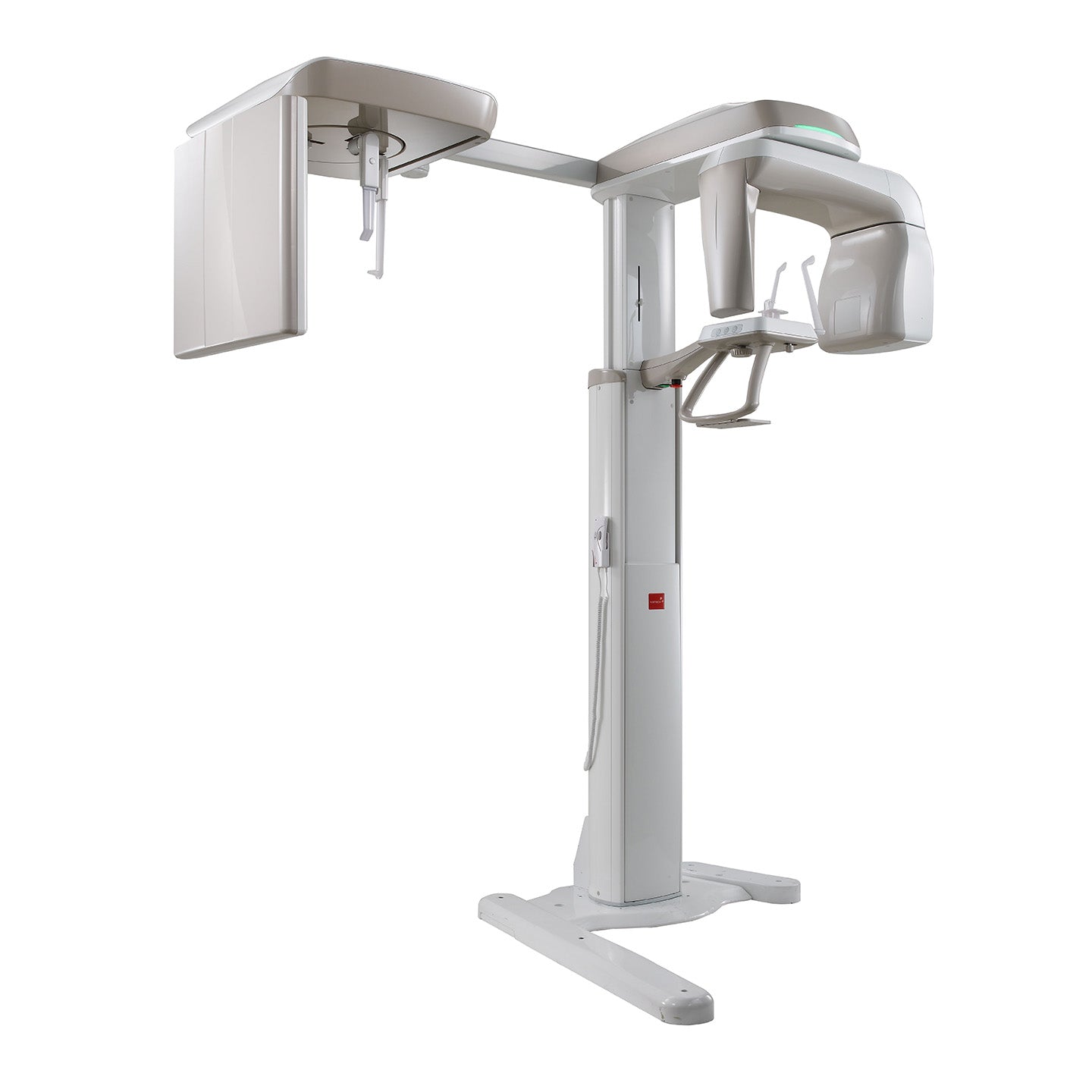
Technical Specifications & Standards
Professional Dental Equipment Guide 2026
Technical Specification Guide: Panoramic & Cephalometric (Pan Ceph) Machine
Target Audience: Dental Clinics & Medical Equipment Distributors
This guide provides a comparative technical overview of Standard and Advanced models of modern Pan Ceph imaging systems, designed for diagnostic precision, workflow efficiency, and compliance with international safety standards.
| Specification | Standard Model | Advanced Model |
|---|---|---|
| Power | Input: 100–240 V AC, 50/60 Hz Max Power Consumption: 1.2 kW X-ray Generator: High-Frequency Inverter (65–90 kVp, 4–16 mA) |
Input: 100–240 V AC, 50/60 Hz Max Power Consumption: 1.5 kW X-ray Generator: High-Frequency Inverter with AEC (60–120 kVp, 2–20 mA, Automatic Exposure Control) |
| Dimensions | Unit: 120 cm (H) × 75 cm (W) × 85 cm (D) Footprint: 0.64 m² Weight: 185 kg (net) |
Unit: 125 cm (H) × 80 cm (W) × 90 cm (D) Footprint: 0.72 m² Weight: 210 kg (net) Includes retractable cephalometric arm and dual-axis patient positioning laser |
| Precision | Image Resolution: 14 LP/mm (line pairs per millimeter) FOV: 14 cm (H) × 19 cm (V) for cephalometric, 520° panoramic rotation Mechanical Tolerance: ±0.5° angular accuracy |
Image Resolution: 20 LP/mm (with digital sensor upgrade) FOV: Adjustable up to 16 cm (H) × 24 cm (V), 3D CBCT optional integration Mechanical Tolerance: ±0.2° angular accuracy with real-time feedback positioning |
| Material | Chassis: Powder-coated steel frame Tube Head Housing: Aluminum alloy with lead shielding (2.0 mm Pb equivalent) Positioning Components: Reinforced ABS plastic and stainless steel joints |
Chassis: Anodized aluminum and composite polymer frame (anti-vibration) Tube Head: Titanium-reinforced housing with 2.5 mm Pb equivalent shielding Positioning: Full stainless steel articulation arms with ceramic bearings |
| Certification | CE Mark (Medical Device Regulation 2017/745) ISO 13485:2016 Certified IEC 60601-1, IEC 60601-2-54 FDA 510(k) Cleared (Class II) |
CE Mark + UKCA ISO 13485:2016 & ISO 14971:2019 (Risk Management) IEC 60601-1-2 (EMC), IEC 62304 (Software) FDA 510(k) Cleared, Health Canada Licensed Compliant with ACR–AAPM Diagnostic Reference Levels |
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
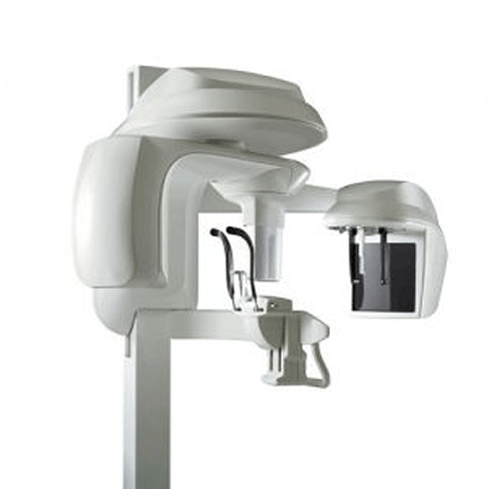
Professional Dental Equipment Sourcing Guide 2026:
Panoramic & Cephalometric (Pan Ceph) Machines from China
Target Audience: Dental Clinic Procurement Managers & Medical Equipment Distributors | Validity: Q1 2026
Strategic Note: Post-2025 regulatory shifts (EU MDR Annex XVI enforcement, updated FDA 510(k) pathways for dental imaging) necessitate stringent supplier vetting. China-sourced Pan Ceph units now require dual certification validation beyond basic CE marking. Prioritize manufacturers with proven NMPA Class III registration.
Step 1: Verifying ISO/CE & Regulatory Credentials (Non-Negotiable for 2026 Compliance)
Chinese manufacturers frequently display generic CE certificates. Demand device-specific documentation:
| Critical Document | 2026 Validation Protocol | Risk of Non-Compliance |
|---|---|---|
| ISO 13485:2016 Certificate | Verify scope explicitly includes “Radiographic Equipment” or “Dental X-ray Systems” via IAF CertSearch. Cross-check certificate # with notified body (e.g., TÜV SÜD, BSI). | Invalid certification voids CE claims; triggers EU customs rejection (MDR Article 10) |
| EU CE Technical File | Require full technical documentation per MDR Annexes II/III. Confirm EU Representative letter (mandatory post-2021) and clinical evaluation report (CER) specific to Pan Ceph imaging. | MDR non-compliance = €20k+ fines per device in EU; FDA import alerts |
| NMPA Registration (China FDA) | Validate Class III registration # on nmpa.gov.cn. Pan Ceph units require NMPA approval (国械注准) for export. | Unregistered units = seizure by Chinese customs; invalidates CE claims |
| Test Reports (IEC 60601-1, -2-63) | Confirm reports from ILAC-MRA accredited labs covering mechanical safety, radiation leakage (≤1mGy/h at 5cm), and software validation. | Device recall risk; liability exposure during clinical use |
Step 2: Negotiating MOQ & Commercial Terms (2026 Market Realities)
Chinese OEMs increasingly enforce minimum order quantities due to raw material volatility (e.g., Gd₂O₂S scintillators). Strategic negotiation framework:
| Term | Recommended Approach | 2026 Market Benchmark |
|---|---|---|
| Base MOQ | Negotiate tiered pricing: 1-2 units (sample) at +15%, 3-5 units at standard rate, 6+ units with 8-12% discount. Avoid suppliers demanding >5 units for first order. | Standard MOQ: 3 units (Pan Ceph combo); 1 unit for standalone cephalometric |
| Payment Terms | Insist on 30% TT advance, 70% against BL copy. Reject 100% upfront. Use LC at sight only with confirmed irrevocable LC. | Typical: 40-60% advance common; 70%+ indicates financial instability |
| Lead Time | Lock in 120-day maximum (includes 30-day pre-shipment QC). Penalize delays at 0.5%/day after Day 120. | Avg. lead time: 100-140 days (2026 supply chain constraints) |
| OEM/ODM Flexibility | Require proof of custom UI development capability (e.g., DICOM 3.0 integration, clinic branding). Verify firmware modification rights in contract. | Top-tier OEMs: 8-12 week customization cycle |
Step 3: Shipping & Logistics (DDP vs. FOB Analysis)
2026 freight volatility demands precise incoterm selection. Critical comparison:
| Parameter | FOB Shanghai | DDP (Your Clinic/Distributor Warehouse) |
|---|---|---|
| Cost Transparency | Hidden costs: Chinese export fees (~$350), THC, documentation surcharges. Final cost +18-25% vs. quote. | All-inclusive pricing. Verified by Carejoy’s 2026 DDP calculator (includes 2026 EU VAT/Duty). |
| Regulatory Risk | Buyer liable for customs clearance errors. Requires local EU rep for MDR compliance. | Supplier handles NMPA export docs, EU customs clearance, and MDR Article 31 rep coordination. |
| Cash Flow Impact | Lower initial quote but multiple payments (freight, insurance, duties). Ties up working capital. | Single invoice. Reduces payment touchpoints by 70% (per 2025 distributor survey). |
| 2026 Recommendation | Only for experienced distributors with in-house customs brokerage. | STRONGLY PREFERRED for clinics & new distributors. Mitigates 2026 EU customs backlog risks. |
Recommended Partner: Shanghai Carejoy Medical Co., LTD
Why Carejoy for Pan Ceph Sourcing in 2026:
- Regulatory Assurance: NMPA Class III Registration (国械注准20232060xxx), ISO 13485:2016 (Scope: Dental X-ray Systems), and EU MDR-compliant CE with TÜV SÜD NB#0123
- MOQ Flexibility: 1-unit samples available; standard MOQ = 2 Pan Ceph units (no standalone cephalometric penalty)
- DDP Expertise: Direct partnerships with DHL & DB Schenker for door-to-door DDP shipping to 45+ countries (avg. transit: 28 days EU, 32 days US)
- Technical Edge: 19 years specializing in dental imaging; OEM firmware development for DICOM 3.0/PACS integration
Baoshan District, Shanghai, China 201900
Core Verification: Request Certificate # SHCJ-PC2026-ISO13485 & EU REP Letter # CAREJOY-EU-REP-2026
Contact: [email protected] | WhatsApp: +86 15951276160
Note: All 2026 Pan Ceph quotes include free radiation safety audit documentation
Actionable Next Steps for Clinics & Distributors
- Pre-qualification: Email suppliers with subject line: “2026 Pan Ceph Verification Request – [Your Clinic/Distributor Name]”. Demand NMPA cert + EU technical file index.
- Sample Protocol: Order 1 unit DDP to validate image quality (test with Rinn XCP sensor) and software workflow before bulk order.
- Contract Clause: Insert: “Seller warrants continuous NMPA/EU MDR compliance through 2028. Non-compliance triggers full refund + logistics costs.”
Disclaimer: This guide reflects 2026 regulatory landscapes. Verify all requirements with local authorities. Shanghai Carejoy is cited as an exemplar of compliant Chinese manufacturing; inclusion does not constitute exclusive endorsement.
Frequently Asked Questions
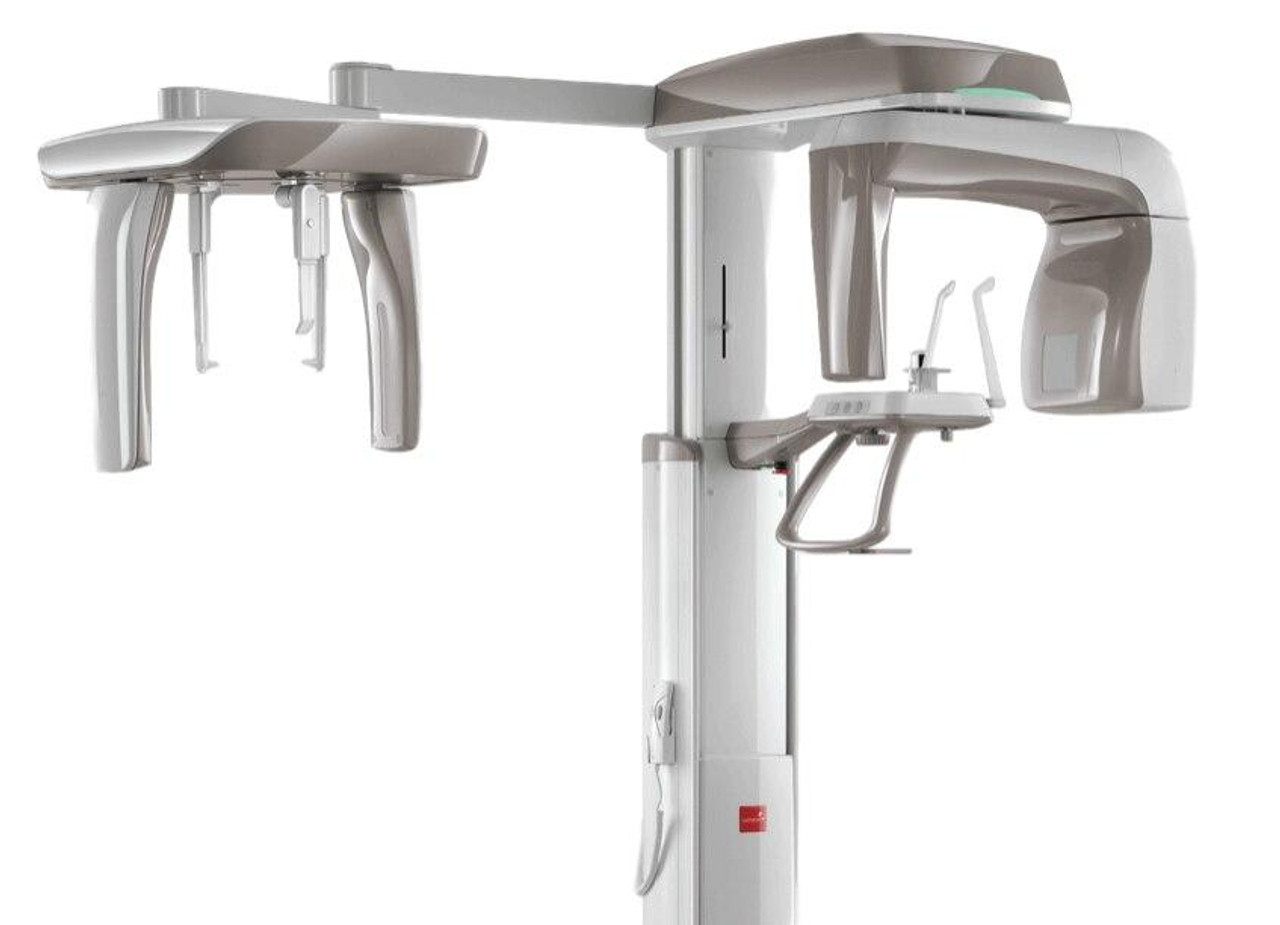
Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Distributors
Product Focus: Panoramic & Cephalometric (Pan Ceph) X-Ray Units
Frequently Asked Questions: Buying a Pan Ceph Machine in 2026
| Question | Professional Answer |
|---|---|
| 1. What voltage requirements should I consider when installing a modern Pan Ceph machine in 2026? | Most advanced Pan Ceph units in 2026 operate on a standard 220–240V AC, 50/60 Hz single-phase power supply. However, high-frequency generators and AI-integrated models may require dedicated circuits with stable voltage and grounding to prevent imaging artifacts and ensure patient safety. Always verify the exact electrical specifications in the technical datasheet and confirm compatibility with local power infrastructure. For regions with unstable grids, integration with a line-interactive UPS (1.5–2 kVA) is recommended to protect sensitive components. |
| 2. Are spare parts for Pan Ceph machines readily available, and what is the expected lead time? | Reputable manufacturers now maintain global spare parts distribution networks with regional hubs to reduce lead times. Critical components such as X-ray tubes, sensors, collimators, and control boards are typically stocked for at least 7–10 years post-discontinuation. Distributors should confirm parts availability and average lead times (usually 3–7 business days for in-stock items) before purchase. We recommend clinics establish a service agreement that includes priority access to spare parts and firmware updates. |
| 3. What does the installation process for a Pan Ceph unit involve, and is professional assistance required? | Installation of a Pan Ceph machine in 2026 is a certified technician-led process involving site preparation, radiation shielding verification, electrical setup, mechanical assembly, software calibration, and DICOM integration. Most manufacturers require factory-trained engineers to perform installation to validate compliance with IEC 60601-2-63 and local radiation safety standards. The process typically takes 1–2 days and includes staff training. Self-installation voids warranty and is not permitted under regulatory guidelines. |
| 4. What is the standard warranty coverage for a Pan Ceph machine, and what does it include? | As of 2026, leading manufacturers offer a comprehensive 3-year parts-and-labor warranty on new Pan Ceph units. This includes coverage for the X-ray generator, imaging sensor, mechanical arms, control system, and software defects. Onsite service response is typically within 48 hours for critical failures. Optional extended warranties (up to 5 years) are available and highly recommended, especially for clinics without in-house service support. Note: Damage from power surges, improper handling, or unauthorized modifications is excluded. |
| 5. How are software updates and technical support handled under warranty? | Warranty agreements now include remote software updates, cybersecurity patches, and AI algorithm enhancements at no additional cost. Manufacturers provide 24/7 technical support via phone, email, and remote diagnostics. Most systems are cloud-connected (with clinic consent) for predictive maintenance alerts and performance monitoring. Ensure your distributor offers multilingual support and SLA-backed response times, particularly for regions with limited service coverage. |
Note: All specifications and services are subject to change based on manufacturer policies and regional regulations. Always request a formal quotation and technical proposal before procurement.
Need a Quote for Pan Ceph Machine?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


