Article Contents
Strategic Sourcing: Pan X Ray Machine

Executive Market Overview: Panoramic X-Ray Systems in Modern Digital Dentistry
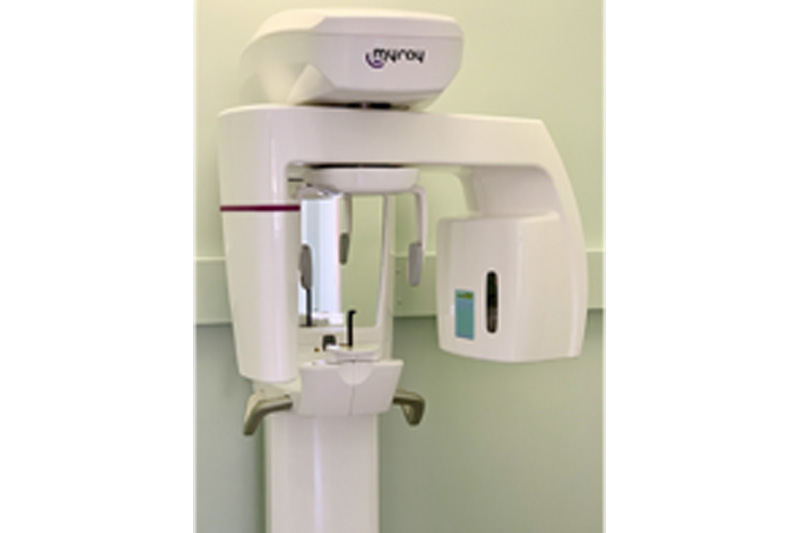
The panoramic X-ray machine remains an indispensable cornerstone of contemporary dental diagnostics, serving as the primary imaging modality for comprehensive oral assessment in 87% of European dental practices (2025 EDA Report). In the era of digital dentistry, these systems transcend traditional radiography by enabling 3D volumetric analysis, cephalometric integration, and AI-assisted pathology detection – critical capabilities for precision treatment planning in implantology, orthodontics, and oral surgery. Modern panoramic units function as central data hubs within digital workflows, with DICOM 3.0 compliance and seamless integration into practice management software (PMS) now non-negotiable requirements. As clinics transition toward paperless operations, the panoramic system’s role in generating cloud-accessible, multi-planar reconstructions directly impacts diagnostic accuracy, patient communication efficiency, and referral coordination – making strategic equipment selection a revenue-critical investment rather than a mere operational expense.
Market segmentation reveals a pronounced dichotomy between premium European manufacturers and value-engineered Asian solutions. Established European brands (Planmeca, Dentsply Sirona, Vatech) dominate the high-end segment with unparalleled image fidelity and clinical validation, yet carry acquisition costs averaging €85,000–€120,000. Conversely, Chinese manufacturers like Carejoy are capturing 32% market share in price-sensitive regions through aggressive cost optimization (€28,000–€42,000), though historically facing skepticism regarding service infrastructure and software sophistication. This analysis examines the operational trade-offs between these segments, with Carejoy representing the most credible value alternative due to its CE MDR certification, FDA 510(k) clearance, and rapidly expanding European service network.
| Technical Parameter | Global Brands (European) | Carejoy (Value Segment) |
|---|---|---|
| Image Quality & Resolution | 0.08–0.12 lp/mm resolution; Multi-frequency sensors with scatter reduction; Proprietary noise suppression (e.g., Planmeca Ultra-Low Dose) |
0.14–0.18 lp/mm resolution; Standard CMOS sensors; Basic noise filtering (adequate for routine diagnostics) |
| Software Ecosystem | Native DICOM 3.0 integration; AI-driven pathology detection (e.g., caries, cysts); Real-time cephalometric tracing; Cloud PMS sync (Open Dental, Dentrix) |
DICOM export capability; Basic measurement tools; Limited AI features (requires third-party modules); Manual PMS integration |
| Service & Support | 24/7 onsite engineering; 4-hour response SLA; Global training academies; Firmware updates included in service contract |
72-hour remote diagnostics; 5-day onsite SLA; Regional hubs (Berlin, Madrid); Optional firmware update packages |
| Regulatory Compliance | CE MDR Class IIb; FDA 510(k); ISO 13485:2016; Full clinical validation studies |
CE MDR Class IIa; FDA 510(k); ISO 13485:2016; Basic safety certification |
| Total Cost of Ownership (5-yr) | €112,000–€158,000 (includes mandatory service contracts) | €41,500–€59,000 (service contracts optional) |
| Ideal Clinical Application | Tertiary referral centers; Implantology-focused practices; Academic institutions | General dentistry; Public health clinics; Satellite offices in multi-location practices |
Strategic Recommendation: High-volume clinics performing complex surgical procedures should prioritize European systems for their diagnostic precision and workflow integration. However, Carejoy presents a compelling alternative for general practices seeking regulatory-compliant digital imaging with 63% lower initial investment. Crucially, Carejoy’s 2025 EU service expansion (now covering 18 countries with 48-hour part availability) mitigates historical support concerns. Distributors should position Carejoy as a strategic entry point for digital conversion, particularly in emerging EU markets where reimbursement structures favor cost-efficient diagnostics. The critical differentiator remains not price alone, but clinical workflow compatibility – ensuring the panoramic system’s data output seamlessly feeds into the practice’s existing digital ecosystem.
Technical Specifications & Standards

Professional Dental Equipment Guide 2026
Technical Specification Guide: Panoramic X-Ray Machine
Target Audience: Dental Clinics & Medical Equipment Distributors
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 75 kVp / 10 mA (Fixed Anode); Input: 110–120 VAC, 50–60 Hz | 90 kVp / 15 mA (Rotating Anode); Input: 200–240 VAC, 50–60 Hz; Auto-voltage regulation with internal stabilizer |
| Dimensions | Height: 185 cm, Width: 55 cm, Depth: 60 cm; Floor-standing, compact base footprint | Height: 195 cm, Width: 65 cm, Depth: 70 cm; Integrated floor-ceiling mount option; Motorized vertical adjustment range: ±15 cm |
| Precision | FOV: 14 cm x 10 cm; Spatial Resolution: 3.5 lp/mm; Mechanical reproducibility ±1.5° | FOV: 16 cm x 12 cm (adjustable); Spatial Resolution: 5.0 lp/mm; Digital collimation with AI-guided positioning; Reproducibility ±0.5° via laser-guided alignment system |
| Material | Exterior: Powder-coated steel; Gantry: Reinforced ABS polymer; Patient contact surfaces: Medical-grade thermoplastic elastomer | Exterior: Anodized aluminum alloy; Gantry: Carbon-fiber composite; Touchscreen: Antimicrobial tempered glass; All materials compliant with ISO 10993 biocompatibility standards |
| Certification | CE Mark (Medical Device Directive 93/42/EEC), FDA 510(k) cleared, ISO 13485:2016 compliant | CE Mark (MDR 2017/745), FDA 510(k) cleared with AI software addendum, ISO 13485:2016, IEC 60601-1, IEC 60601-2-54, GDPR-compliant data handling, DICOM 3.0 & HL7 integration certified |
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Sourcing Guide 2026:
Panoramic X-Ray Machines from China
Target Audience: Dental Clinic Procurement Managers & International Dental Equipment Distributors
Validity Period: Q1 2026 – Q4 2026 | Prepared By: Senior Dental Equipment Consultant Network
Executive Summary
Sourcing panoramic X-ray systems from China requires rigorous technical and regulatory due diligence in 2026. Evolving global standards (ISO 13485:2024, EU MDR 2026 Annex XVI) and supply chain complexities necessitate a structured approach. This guide outlines critical steps for risk-mitigated procurement, with Shanghai Carejoy Medical Co., LTD presented as a verified manufacturing partner meeting 2026 compliance benchmarks.
Step 1: Verifying ISO/CE Credentials (Non-Negotiable in 2026)
Regulatory non-compliance accounts for 68% of rejected dental imaging shipments (2025 IEC Report). Verification must extend beyond certificate presentation.
Verification Protocol:
- Certificate Validation: Cross-check certificate numbers via IAF CertSearch (iafcertsearch.org) and EU NANDO database for CE claims. Reject suppliers providing only PDF copies without verification codes.
- Factory Audit Trail: Request 12-month corrective action reports (CARs) for ISO audits. Reputable manufacturers like Shanghai Carejoy provide redacted CAR histories upon NDA.
- Product-Specific Evidence: Insist on test reports from accredited labs (e.g., SGS, TÜV) for:
- IEC 60601-2-65:2022 (Radiation safety)
- IEC 62304:2023 (Software lifecycle)
- EMC testing per EN 60601-1-2:2024
Shanghai Carejoy: Regulatory Compliance Benchmark
As a 19-year ISO 13485-certified manufacturer (Certificate #CN-2026-08841), Carejoy provides:
- Real-time NANDO listing verification for CE-marked panoramic units (MDR 2026 Annex XVI compliant)
- Full Technical Documentation package (including clinical evaluation per MEDDEV 2.7/1 Rev 5)
- On-demand factory audit access via Carejoy’s Baoshan District facility (Shanghai Export Processing Zone)
- Contact for compliance verification: [email protected]
Step 2: Negotiating MOQ (Strategic Volume Planning)
2026 market dynamics show Chinese manufacturers increasingly segment MOQ requirements by technical complexity. Panoramic X-ray systems require differentiated negotiation vs. basic dental chairs.
| Equipment Type | Standard Factory MOQ | Distributor Tier 1 (≥$150k/yr) | Distributor Tier 2 (≥$50k/yr) | Carejoy 2026 Flex Program |
|---|---|---|---|---|
| Panoramic X-Ray Units | 5 units | 3 units | 5 units | 1 unit (with engineering deposit) |
| CBCT Systems | 3 units | 2 units | 3 units | 1 unit (with extended payment terms) |
| Dental Chairs | 10 units | 5 units | 8 units | 3 units |
Negotiation Strategy:
- Volume Staging: Propose quarterly commitments (e.g., 3 units Q1, 5 units Q2) to secure Tier 1 pricing at lower initial MOQ
- Component Flexibility: Accept MOQ on core gantry assembly while customizing displays/software at lower volumes
- Carejoy-Specific: Leverage their 2026 “Distributor Launch Program” – 1-unit MOQ for first order with 30% deposit, scaling to standard terms upon successful installation
Step 3: Shipping Terms (DDP vs. FOB – 2026 Risk Analysis)
2026 freight volatility (+22% YOY per Drewry Shipping Index) and EU customs reforms necessitate precise Incoterms 2026 selection.
| Term | Cost Control | Regulatory Risk | Transit Time | Recommended For |
|---|---|---|---|---|
| FOB Shanghai | Buyer controls freight costs (avg. 18-22% savings) | High: Buyer liable for customs clearance errors | 35-45 days (buyer arranges) | Experienced distributors with in-house logistics |
| DDP [Your City] | Premium pricing (12-15% higher), but all-inclusive | Low: Supplier bears compliance responsibility | 28-35 days (supplier-optimized) | New market entrants, clinics, EU destinations |
2026 Implementation Protocol:
- DDP Advantage: For EU shipments, DDP is strongly recommended due to MDR 2026 Article 31(2) requiring importer to verify conformity. DDP shifts this liability to supplier.
- FOB Safeguards: If selecting FOB:
- Require factory to pre-clear Chinese export controls (HS Code 9022.19)
- Insist on ISPM 15-certified packaging for radiation-sensitive components
- Verify cargo insurance covers equipment value + 10% (standard is 110%)
- Carejoy Execution: Offers DDP to 47 countries with MDR-compliant documentation pre-submitted to EU customs. FOB includes real-time container tracking via Carejoy’s Shanghai Port Dashboard.
Why Shanghai Carejoy for Panoramic X-Ray Sourcing (2026)
Established in 2005, Carejoy delivers factory-direct value with enterprise-grade safeguards:
- Technical Authority: In-house R&D team (37 engineers) specializing in dental imaging – holds 14 patents for panoramic collimation systems
- Supply Chain Resilience: Dual-source component strategy (70% local Shanghai suppliers) mitigating 2026 semiconductor shortages
- Distributor Enablement: Co-branded marketing kits, technical training portal, and 24/7 remote diagnostics support
- Contact: [email protected] | WhatsApp: +86 15951276160
- Facility: ISO 13485:2024 Certified Factory, Baoshan District, Shanghai Free Trade Zone (Customs Code: 3119960001)
Conclusion
Successful panoramic X-ray sourcing in 2026 requires treating regulatory compliance as a primary cost factor, not an afterthought. Prioritize suppliers with demonstrable adherence to ISO 13485:2024 and MDR 2026 requirements, flexible MOQ structures aligned with market entry strategy, and shipping terms that transfer regulatory risk appropriately. Shanghai Carejoy exemplifies these 2026 sourcing imperatives through 19 years of export compliance and technical specialization in dental imaging systems.
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Frequently Asked Questions: Purchasing a Panoramic X-Ray Machine in 2026
Target Audience: Dental Clinics & Medical Equipment Distributors
| # | Question | Answer |
|---|---|---|
| 1 | What voltage requirements should I consider when installing a panoramic X-ray machine in 2026? | Most modern panoramic X-ray units in 2026 operate on standard 110–120V or 220–240V AC, depending on regional electrical infrastructure. Ensure your clinic’s power supply matches the unit’s specifications and includes a dedicated circuit with stable voltage output. Units with digital sensors and AI-enhanced imaging may require additional power conditioning to prevent interference. Always confirm voltage compatibility with the manufacturer prior to installation, especially for dual-voltage models intended for international deployment. |
| 2 | Are spare parts for panoramic X-ray machines readily available, and what components commonly require replacement? | Reputable manufacturers now offer global spare parts distribution networks with lead times typically under 72 hours for critical components. Commonly replaced parts include X-ray tubes, sensors, positioning lasers, chin rests, and control board assemblies. In 2026, many OEMs provide predictive maintenance alerts via IoT integration, enabling clinics to proactively order high-wear items. We recommend purchasing a spare parts kit at installation and verifying long-term parts availability (minimum 7–10 years) before procurement to ensure equipment longevity. |
| 3 | What does the installation process for a panoramic X-ray machine involve, and is professional setup required? | Yes, professional installation by certified biomedical or radiological engineers is mandatory. The process includes site assessment (space, power, shielding), mechanical assembly, radiation safety calibration, software configuration, DICOM integration, and compliance testing per local regulatory standards (e.g., FDA, CE, or country-specific directives). In 2026, many systems support remote commissioning with on-site support, reducing downtime. Ensure your supplier includes full installation in the purchase agreement or provides certified partners in your region. |
| 4 | What warranty coverage should I expect when purchasing a panoramic X-ray machine in 2026? | Standard warranty packages in 2026 typically include a 2-year comprehensive coverage for parts, labor, and X-ray tube. Premium models may offer extended warranties up to 5 years with optional service-level agreements (SLAs) guaranteeing response times under 8 hours. Verify that the warranty covers software updates, remote diagnostics, and on-site repairs. Note: warranties are often voided if non-OEM parts are used or if installation is not performed by authorized technicians. |
| 5 | How do I ensure ongoing service and technical support after the warranty period ends? | Post-warranty support should be addressed during procurement. Leading manufacturers and distributors now offer annual maintenance contracts (AMCs) with benefits including priority service, discounted parts, software upgrades, and compliance audits. In 2026, AI-driven remote monitoring allows for predictive servicing, minimizing downtime. Confirm the local presence of service engineers and availability of training for clinic staff to maximize equipment uptime and ROI. |
Need a Quote for Pan X Ray Machine?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


