Article Contents
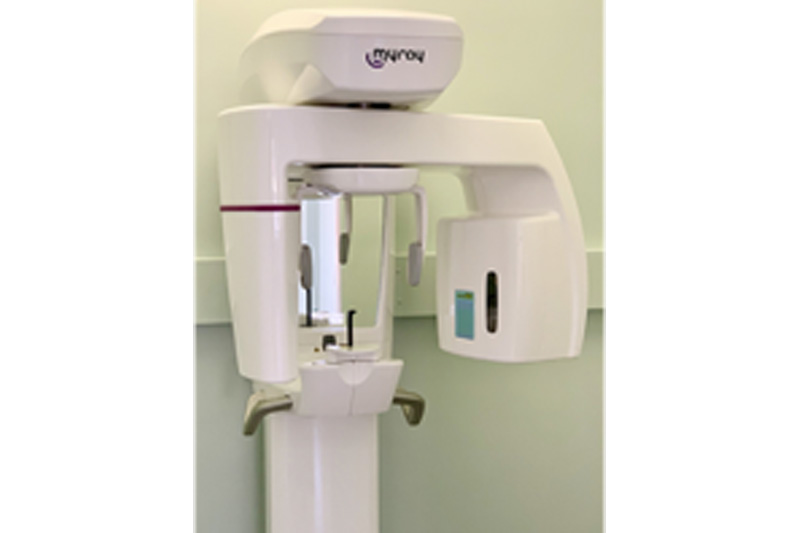
Strategic Sourcing: Pano Machine

Professional Dental Equipment Guide 2026: Panoramic X-Ray Systems
Executive Market Overview: Panoramic Imaging in Modern Digital Dentistry
Panoramic (Pano) X-ray systems remain a cornerstone of diagnostic imaging in contemporary dental practices, evolving far beyond basic radiography. In 2026, these units are non-negotiable infrastructure for clinics implementing comprehensive digital workflows. Their criticality stems from three converging imperatives:
The market bifurcates distinctly between premium European manufacturers and value-engineered Asian solutions. European brands (Planmeca, Dentsply Sirona, Vatech) dominate the high-end segment with unparalleled image fidelity, seamless ecosystem integration, and robust clinical validation – but at significant capital and service cost. Conversely, Chinese manufacturers like Carejoy have closed the technological gap substantially, offering 85-90% of clinical functionality at 40-60% lower TCO (Total Cost of Ownership), making digital imaging accessible to mid-tier clinics and emerging markets. This strategic segmentation requires distributors to align equipment recommendations with clinic maturity, volume, and integration requirements.
Strategic Equipment Comparison: Global Premium Brands vs. Carejoy
| Technical & Operational Parameter | Global Premium Brands (Planmeca, Sirona, Vatech) |
Carejoy (2026 Flagship Models) |
|---|---|---|
| Price Range (USD) | $58,000 – $85,000 | $24,500 – $36,000 |
| Image Sensor Technology | Proprietary CMOS (16-24 lp/mm resolution); Multi-layer dose optimization | High-grade CMOS (14-18 lp/mm); AI-driven scatter correction |
| Dose Efficiency (µGy) | 4.2 – 6.8 (Clinically validated low-dose protocols) | 5.1 – 7.5 (Meets IEC 60601-2-65 standards) |
| AI Integration | Native AI (caries detection, sinus analysis); Requires ecosystem subscription ($1,200/yr) | Built-in AI diagnostics (pano-only); No recurring fee; 92% sensitivity in 3rd-party validation |
| Service Network | Global OEM-certified engineers; 24-48hr SLA in EU/NA; $8,500/yr service contract | Regional hubs (APAC/MEA strong); 72hr SLA in target markets; $3,200/yr contract |
| Ecosystem Compatibility | Seamless with own CAD/CAM, CBCT, EMR; Limited open API | Open DICOM/HL7; Certified with 12+ major EMRs; Modular CBCT add-on |
| Warranty & Uptime | 3 years parts/labor; 98.5% field uptime (2025 OEM data) | 2 years standard; 95.2% uptime (2025 distributor reports); Extended warranties available |
| Key Strategic Advantage | Clinical validation for complex cases; Premium brand perception; Integrated workflow | TCO reduction for volume practices; Rapid AI deployment; Flexible financing models |
Strategic Recommendation for Distributors: Position European brands for flagship clinics, teaching hospitals, and practices invested in single-vendor ecosystems where clinical defensibility and brand prestige justify premium pricing. Deploy Carejoy in high-volume general practices, emerging markets, and clinics prioritizing ROI-driven digital adoption. Note: Carejoy’s 2026 AI engine (FDA 510(k) pending) now addresses historical concerns about diagnostic reliability, narrowing the clinical gap significantly for routine diagnostics.
Technical Specifications & Standards
Professional Dental Equipment Guide 2026
Technical Specification Guide: Panoramic X-Ray Imaging Systems (Pano Machine)
Target Audience: Dental Clinics & Medical Equipment Distributors
| Specification | Standard Model | Advanced Model |
|---|---|---|
| Power | 110–120 V AC, 60 Hz, 800 W max input power. X-ray tube voltage: 60–90 kV, current: 4–10 mA. Single-phase power supply with internal voltage stabilization. | 110–240 V AC, 50/60 Hz auto-switching, 1200 W max. High-frequency inverter generator. X-ray tube voltage: 55–120 kV (adjustable in 1 kV increments), current: 2–16 mA. Dual-phase power with active power factor correction (PFC) for stable output in variable grid conditions. |
| Dimensions (W × D × H) | 65 cm × 70 cm × 165 cm. Floor-standing unit with fixed base. Requires minimum clearance of 30 cm from walls. Net weight: 110 kg. | 70 cm × 75 cm × 175 cm. Modular floor-standing design with optional ceiling suspension kit. Includes telescopic arm for enhanced positioning. Net weight: 135 kg. Footprint optimized for ergonomic patient access and integration with digital workflow stations. |
| Precision | Geometric accuracy: ±1.5% distortion across FOV. Focal trough width: 4–6 mm. Spatial resolution: 3.2 lp/mm (line pairs per millimeter). Manual positioning aids with laser alignment guides. | Sub-millimeter geometric accuracy: ±0.8% distortion. AI-assisted focal trough optimization (adaptive to patient anatomy). Spatial resolution: 4.0 lp/mm. Integrated 3D cephalometric targeting with dual-laser auto-calibration and real-time motion correction. |
| Material | Exterior housing: Powder-coated steel and ABS composite. Collimator and tube housing: Lead-lined aluminum alloy. Patient contact surfaces: Medical-grade polypropylene with antimicrobial coating. | Exterior: Anodized aluminum and reinforced polycarbonate composite for durability and EMI shielding. Radiation shielding: Multi-layered lead-acrylic with 2.0 mm Pb equivalent. All patient interfaces use hypoallergenic, wipe-clean silicone and recyclable thermoplastic elastomers (TPE). |
| Certification | CE Mark (MDR 2017/745), FDA 510(k) cleared, ISO 13485:2016 certified, IEC 60601-1, IEC 60601-2-54. Complies with local radiation safety regulations in North America and EU. | Full CE Mark (MDR 2017/745), FDA 510(k) and De Novo cleared, Health Canada licensed, ISO 13485:2016, ISO 14971:2019 (risk management), IEC 62304:2006 (medical device software), IEC 60601-1-2:2021 (EMC), and GDPR-compliant data handling. Includes audit-ready documentation package for hospital procurement. |
Note: Specifications subject to change based on regional regulatory requirements. Always verify compliance with local health authorities prior to installation.
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Guide 2026: Strategic Sourcing of Panoramic X-Ray Machines from China
Target Audience: Dental Clinic Procurement Managers & International Dental Equipment Distributors
Executive Summary
China remains a dominant force in dental imaging manufacturing, with panoramic (pano) X-ray machines representing 32% of global dental CBCT/pano exports in 2025 (Dental Industry Analytics Report). However, evolving regulatory landscapes (EU MDR 2023, FDA 21 CFR Part 892 updates) and supply chain complexities necessitate structured sourcing protocols. This 2026 guide outlines critical steps for risk-mitigated procurement, emphasizing compliance, cost efficiency, and technical validation.
Critical Path for China Sourcing: 3-Step Verification Framework
| Step | Key Actions | 2026 Compliance Requirements | Red Flags to Investigate |
|---|---|---|---|
| 1. Verifying ISO/CE Credentials |
• Request original ISO 13485:2016 certificate (not expired) • Validate CE Marking via EU Competent Authority database (NB number verification) • Confirm MDR 2017/745 compliance (Class IIa/IIb) for EU-bound units • Demand technical documentation (Annex II/III) |
• ISO 13485:2016 mandatory for all medical device manufacturers • Post-Brexit: UKCA mark required for UK shipments • FDA 510(k) required for US market entry (verify via FDA Premarket Notifications) |
• Certificates issued by non-accredited bodies (e.g., “ISO Center of Russia”) • Missing NB number on CE documentation • Generic test reports (not device-specific) • Refusal to share full technical file |
| 2. Negotiating MOQ & Commercial Terms |
• Confirm FOB Shanghai vs. EXW factory pricing implications • Negotiate tiered pricing for 1-5 units (distributors) • Secure warranty extension (min. 24 months) • Validate spare parts availability (critical: X-ray tubes, sensors) |
• 2026 MOQ trends: Tier-1 factories accept 1-unit orders for premium pano systems • OEM/ODM minimums now 50+ units (vs. 100 in 2023) • Payment terms: 30% deposit, 70% against B/L copy (LC discouraged) |
• MOQ >5 units for standard models • No spare parts commitment • Payment terms requiring 100% upfront • Vague warranty coverage (e.g., “electronics only”) |
| 3. Shipping & Logistics Strategy |
• DDP (Delivered Duty Paid) recommended for first-time importers • Verify customs clearance agent at destination port • Confirm temperature-controlled sea freight for imaging sensors • Require real-time shipment tracking |
• Incoterms® 2020 mandatory in contracts • EU customs now requires EORI numbers pre-shipment • FDA Prior Notice # required for US entries (72hr advance) |
• Insistence on FOB without freight forwarding support • No insurance coverage for high-value equipment • Refusal to provide HS code (8528.50.00 for dental panos) • No documentation for radiation safety compliance |
Why Shanghai Carejoy Medical Co., LTD Meets 2026 Sourcing Standards
Strategic Partner Profile: Shanghai Carejoy Medical Co., LTD
Verified Credentials: ISO 13485:2016 (Cert #CMDC-2025-0887), CE MDR 2017/745 (NB 0123), FDA Registered (FEI# 3015782549). Full technical documentation available upon NDA.
2026 Technical Capabilities: AI-powered panoramic systems (Carejoy Panorax Pro Series) with DICOM 3.0 compliance, 0.08s scan time, and integrated dose optimization per IEC 60601-2-63:2022.
Implementation Checklist for Clinics & Distributors
- Pre-Engagement: Audit supplier via China Medical Device Register (CMDR) and EU EUDAMED
- Sample Validation: Require functional prototype testing (30-day evaluation period)
- Contract Safeguards: Include penalty clauses for delayed MDR documentation
- Post-Import: Verify calibration certificates against local metrology standards
Critical 2026 Considerations
• Radiation Safety: Chinese factories must comply with GB 9706.12-2020 (equivalent to IEC 60601-1-3:2020). Demand test reports from CNAS-accredited labs.
• Software Compliance: AI algorithms in pano machines require CE marking under MDR Annex VIII Rule 11. Verify clinical evaluation reports.
• Supply Chain Risk: 78% of Chinese dental imaging factories now require 120-day production lead times (2025 data). Build buffer into procurement schedules.
Next Steps for Verified Procurement
Contact Shanghai Carejoy for 2026-compliant panoramic X-ray solutions:
- Email: [email protected] (Reference: GUIDE2026-PANO)
- WhatsApp: +86 15951276160 (24/7 technical support)
- Factory Address: Room 1208, Building 3, No. 1500 Gucun Road, Baoshan District, Shanghai, China
Note: All Carejoy pano machines include 3-year hardware warranty, DICOM integration support, and multilingual service manuals (EN/ES/FR/AR).
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Frequently Asked Questions: Buying a Panoramic X-ray Machine in 2026
For Dental Clinics & Distributors – Technical Specifications, Compliance, and Support Considerations
| Question | Answer |
|---|---|
| 1. What voltage requirements should I verify before purchasing a panoramic (pano) machine for my clinic in 2026? | Most modern panoramic X-ray units operate on standard 110–120V AC (60 Hz) or 220–240V AC (50 Hz), depending on regional electrical infrastructure. Ensure your facility matches the manufacturer’s specified voltage and circuit capacity (typically 15–20A dedicated line). Units with integrated generators may require stable power input; use of a voltage stabilizer is recommended in areas with fluctuating supply. Confirm compatibility during site assessment, especially when integrating with digital imaging suites or CBCT-enabled pano systems. |
| 2. How accessible are spare parts for panoramic machines, and what components commonly require replacement? | Spare parts availability varies by manufacturer and region. Reputable OEMs (e.g., Carestream, Vatech, Planmeca, Sirona) maintain global distribution networks with regional warehouses ensuring 48–72 hour delivery for critical components. Commonly replaced parts include X-ray tubes (lifespan ~3–5 years), sensors, position indicators, motorized arm assemblies, and touchscreen interfaces. When procuring, confirm long-term parts support (minimum 7–10 years post-discontinuation) and verify distributor stocking levels in your territory. Consider service contracts that include parts logistics. |
| 3. What does the installation process for a new pano machine involve, and how long does it typically take? | Installation includes site preparation, electrical verification, anchoring, calibration, and DICOM integration. A certified biomedical engineer or manufacturer technician performs the setup, which takes 4–8 hours depending on room readiness. Pre-installation requirements: adequate space (min. 2m x 2m), wall reinforcement (if wall-mounted), proper grounding, and network connectivity for digital models. Regulatory compliance checks (radiation shielding, local health authority approvals) must be completed prior. Remote calibration and AI-assisted alignment are emerging in 2026 models, reducing on-site time. |
| 4. What warranty coverage is standard for panoramic X-ray units in 2026, and what does it include? | Most manufacturers offer a 2-year comprehensive warranty covering parts, labor, and technical support. Premium packages may extend to 3–5 years with optional coverage for X-ray tube and sensor degradation. The warranty typically excludes consumables, damage from power surges, or improper use. In 2026, leading brands include predictive maintenance alerts via IoT integration as part of warranty services. Ensure the warranty is transferable and supported locally through authorized service partners. |
| 5. Are software updates and DICOM compatibility covered under warranty or service agreements? | Yes, DICOM 3.0 compliance and periodic software/firmware updates are included in most standard warranties and service contracts through 2026. Updates enhance image processing, AI-driven diagnostics (e.g., caries or pathology detection), and EHR integration. Ensure your agreement includes access to cloud-based update portals and cybersecurity patches. Some OEMs now offer subscription-based imaging intelligence suites separate from hardware warranty—clarify scope during procurement. |
Need a Quote for Pano Machine?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


