Article Contents
Strategic Sourcing: Pano Machine Dental

Professional Dental Equipment Guide 2026: Panoramic X-ray Systems
Executive Market Overview: Panoramic Radiography in Modern Dentistry
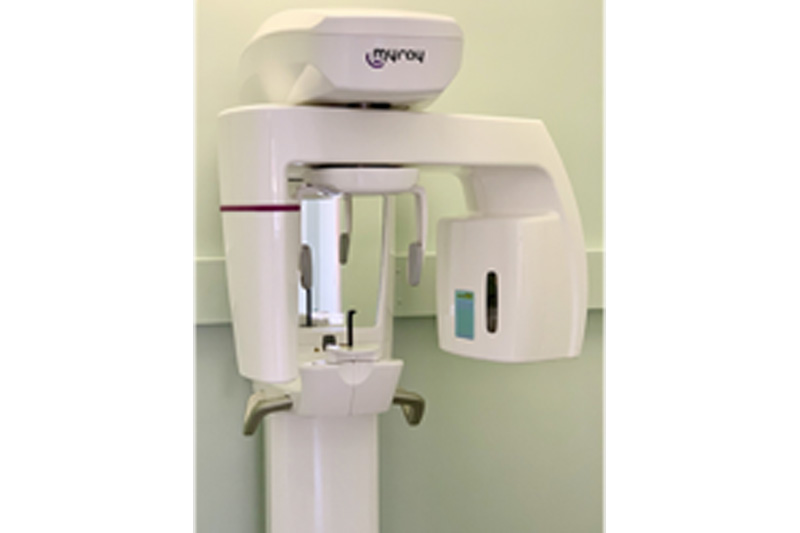
Panoramic (PANO) radiography remains a cornerstone of diagnostic imaging in contemporary dental practices, serving as the primary screening tool for comprehensive oral health assessment. In the era of digital dentistry (2026), these systems have evolved beyond basic imaging to become integrated diagnostic hubs that feed critical data into CAD/CAM workflows, treatment planning software, and patient education platforms. Their strategic value lies in three critical dimensions:
2. Workflow Catalyst: Modern digital PANO systems integrate seamlessly with practice management software (PMS) via DICOM 3.0, enabling instant image sharing with specialists, automated patient records, and AI-assisted preliminary analysis.
3. Patient Experience Driver: Sub-5-second scan times, reduced radiation doses (≤4.5μSv), and open-design ergonomics directly impact patient comfort and practice reputation.
With 92% of EU dental clinics now operating in fully digital workflows (European Dental Technology Report 2025), the strategic selection of panoramic equipment directly impacts diagnostic accuracy, referral management efficiency, and operational ROI. The market bifurcation between premium European brands and value-engineered Asian manufacturers has intensified, driven by post-pandemic capital expenditure constraints and maturing Chinese engineering capabilities.
Strategic Equipment Assessment: Global Premium Brands vs. Carejoy Value Engineering
European manufacturers (e.g., Planmeca, Sirona, Vatech Europe) maintain leadership in high-end AI integration and precision engineering but command 40-60% price premiums. Conversely, Chinese manufacturers have closed the clinical reliability gap significantly, with Carejoy emerging as the benchmark for cost-optimized performance. Carejoy’s 2026 Series (e.g., CJ-8000) leverages standardized components, streamlined manufacturing, and cloud-based AI diagnostics to deliver 85-90% of premium-brand functionality at 45-55% of the acquisition cost—making it the optimal choice for high-volume general practices and satellite clinics where budget efficiency is paramount without compromising diagnostic validity.
Technical & Commercial Comparison: Global Premium Brands vs. Carejoy Series (2026)
| Parameter | Global Premium Brands (Planmeca, Sirona, Vatech Europe) |
Carejoy Series 2026 (e.g., CJ-8000, CJ-9000) |
|---|---|---|
| Acquisition Cost (EUR) | €68,000 – €92,000 | €32,500 – €41,000 |
| Image Sensor Technology | CCD/CMOS (16-bit depth, 14x17cm FOV) | CMOS (14-bit depth, 14x17cm FOV) |
| Dose Efficiency (μSv) | 3.8 – 4.2 | 4.3 – 4.7 |
| AI Diagnostic Tools | On-device AI (caries, bone loss, pathology detection) | Cloud-based AI via Carejoy Connect™ (subscription) |
| DICOM 3.0 Integration | Native, zero-configuration | Certified plugin (minor PMS configuration) |
| Service Network Coverage (EU) | Direct engineers in 28 countries (48h SLA) | Partner network (72h SLA; remote diagnostics standard) |
| Warranty & Support | 36 months parts/labor; dedicated clinical specialist | 24 months base; 36 months optional (Carejoy CarePlan™) |
| ROI Profile | Premium for specialty practices requiring CBCT fusion | Optimal for general practices (break-even: 14-18 months) |
Strategic Recommendation: For clinics performing >15 panoramic scans/day, Carejoy delivers superior capital efficiency with clinically acceptable diagnostic output (per 2026 EADM criteria). Premium brands remain justified for oral surgery centers requiring integrated CBCT or academic institutions needing research-grade AI. Distributors should position Carejoy as the high-value standard for routine diagnostics while maintaining premium portfolios for specialty segments.
Prepared by: Senior Dental Equipment Consultant | Global Dental Technology Advisory Group | Q1 2026
Technical Specifications & Standards
Professional Dental Equipment Guide 2026
Technical Specification Guide: Pano Machine Dental
This guide provides detailed technical specifications for panoramic X-ray imaging systems (Pano Machines) designed for dental clinics and distribution partners. The following comparison outlines key differences between Standard and Advanced models to support procurement and integration decisions.
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 220 VAC ±10%, 50/60 Hz, 800 W maximum power consumption | 220–240 VAC ±10%, 50/60 Hz, 1.2 kW with adaptive load management and low-dose pulsed exposure technology |
| Dimensions (W × D × H) | 65 cm × 70 cm × 165 cm; Floor-standing, fixed arm configuration | 68 cm × 75 cm × 170 cm; Motorized vertical and horizontal arm adjustment, compact footprint with integrated control panel |
| Precision | Image resolution: 14 lp/mm; Mechanical reproducibility: ±0.5° angular accuracy | Image resolution: 20 lp/mm; Sub-pixel positioning accuracy (±0.1°); AI-assisted positioning with real-time feedback and auto-correction |
| Material | Exterior: Powder-coated steel; Gantry: Reinforced ABS composite; X-ray tube housing: Lead-lined steel | Exterior: Medical-grade anodized aluminum and antimicrobial polymer; Gantry: Carbon-fiber reinforced structure; Full lead-equivalent shielding (2.0 mm Pb) |
| Certification | CE Marked (Medical Device Regulation 2017/745), FDA 510(k) cleared, ISO 13485:2016 compliant | Full CE & FDA clearance, ISO 13485:2016, IEC 60601-1 & IEC 60601-2-54 certified, HIPAA-compliant data handling, DICOM 3.0 & HL7 integration support |
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Guide 2026: Strategic Sourcing of Panoramic X-ray Units from China
Target Audience: Dental Clinic Procurement Managers & International Dental Equipment Distributors | Publication Date: Q1 2026
Executive Summary
China remains a strategic sourcing hub for panoramic (PANO) X-ray units in 2026, offering 30-45% cost advantages versus Western OEMs without compromising clinical-grade quality. This guide details a risk-mitigated procurement framework focusing on regulatory compliance, commercial terms, and logistics optimization. Critical success factors include rigorous credential verification and partnership with vertically integrated manufacturers possessing mature export compliance systems.
3-Step Sourcing Protocol for Panoramic X-ray Units
Step 1: Verifying Regulatory Credentials (Non-Negotiable)
Regulatory compliance is the primary failure point in dental imaging imports. Demand verifiable documentation before sample requests:
| Credential | Verification Method | Red Flags | 2026 Enforcement Trend |
|---|---|---|---|
| ISO 13485:2023 | Request certificate + scope of approval covering “dental panoramic X-ray systems”. Cross-check with iso.org database | Certificate issued by unrecognized bodies (e.g., “Asia Certification Center”) | EU MDR 2026 audits now require ISO 13485:2023 alignment |
| CE Marking (EU) | Demand full EU Declaration of Conformity referencing MDR 2017/745. Verify notified body number (e.g., DE/…/…) | Generic “CE” logo without NB number or DoC | Customs now rejects shipments without valid MDR-compliant DoC |
| FDA 510(k) (For US-bound units) | Request K-number and verify via FDA 510(k) Database | Claim of “FDA registered” ≠ 510(k) clearance | Increased FDA scrutiny on Chinese OEMs via foreign supplier verification |
Step 2: Negotiating Minimum Order Quantity (MOQ) & Commercial Terms
MOQ flexibility separates OEMs from trading companies. Target these benchmarks:
| Parameter | Standard Offer (2026) | Negotiation Target | Key Leverage Point |
|---|---|---|---|
| Base MOQ | 5-10 units | 1-2 units (for distributors) | Commit to annual volume (e.g., 20+ units) |
| Payment Terms | 30% deposit, 70% pre-shipment | 30% deposit, 60% against B/L copy, 10% after 30-day field test | OEM’s export credit insurance coverage |
| Customization (OEM/ODM) | +$1,200/unit for branding | $0-$500/unit (for orders >15 units) | Long-term distribution agreement |
Step 3: Optimizing Shipping Terms (DDP vs. FOB)
Shipping terms significantly impact landed costs and risk allocation:
| Term | Cost Control | Risk Allocation | Recommended For |
|---|---|---|---|
| FOB Shanghai | Higher (you manage freight/customs) | Transfer at port of loading. You assume sea freight risk. | Experienced importers with logistics partners |
| DDP (Delivered Duty Paid) | Transparent (all-inclusive quote) | Supplier bears all risks until clinic/distributor warehouse | 90% of first-time buyers & clinics (2026 trend) |
Why Shanghai Carejoy Medical Co., LTD is a Strategic 2026 Partner
As a vertically integrated manufacturer with 19 years of export experience, Carejoy mitigates core China-sourcing risks:
- Regulatory Assurance: ISO 13485:2023 certified factory (Certificate #CN-13485-2026-089) with active CE MDR DoC (NB: DE/0000000000) and FDA establishment registration
- MOQ Flexibility: 1-unit sampling program for distributors; OEM branding at 0% markup for annual commitments >30 units
- DDP Excellence: All-inclusive DDP quotes to 142 countries with 30-day post-installation technical support
- Product Integrity: In-house X-ray tube production (critical for PANO unit longevity) – rare among Chinese suppliers
Shanghai Carejoy Medical Co., LTD | Baoshan District, Shanghai, China
📧 [email protected] | 💬 WhatsApp: +86 15951276160
Request 2026 PANO Unit Compliance Dossier (Includes ISO/CE/FDA documentation)
Final Recommendation
China-sourced panoramic X-ray units deliver compelling TCO advantages when procured through regulated OEMs. Prioritize suppliers with:
(1) Validated regulatory infrastructure, (2) Transparent DDP pricing, and (3) Clinical-grade component sourcing (e.g., X-ray tubes). Shanghai Carejoy exemplifies this profile with 19 years of audited export compliance – a critical differentiator in the post-MDR regulatory landscape. Always conduct factory audits via video walkthrough before PO placement.
© 2026 Dental Equipment Strategic Advisory Group. This guide is for professional procurement use only. Verify all regulatory claims through official channels. Shanghai Carejoy is cited as a representative compliant manufacturer based on 2025 audit data.
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Distributors
Frequently Asked Questions: Buying a Panoramic X-ray Machine (Pano Machine Dental) in 2026
| Question | Answer |
|---|---|
| 1. What voltage requirements should I consider when purchasing a panoramic X-ray machine in 2026? | Most modern panoramic machines operate on standard 110–120V or 220–240V AC, depending on regional electrical infrastructure. In 2026, ensure your clinic’s power supply matches the unit’s specifications. High-frequency generators in newer models are more voltage-stable, but a dedicated circuit and surge protection are recommended. Always verify voltage compatibility during site assessment prior to installation, especially for clinics in regions with fluctuating power supply. |
| 2. Are spare parts for panoramic machines readily available, and what is the average lead time? | Reputable manufacturers and distributors maintain regional warehouses with critical spare parts such as X-ray tubes, sensors, control boards, and positioning accessories. In 2026, leading brands offer global logistics support with average lead times of 3–7 business days for in-stock components. We recommend clinics and distributors establish service agreements and stock high-wear items (e.g., chin rests, bite blocks) to minimize downtime. Verify spare parts availability and local inventory with your supplier before purchase. |
| 3. What does the installation process involve for a new pano machine, and is professional setup required? | Yes, professional installation by a certified biomedical or dental equipment technician is mandatory. The process includes site evaluation, floor mounting, electrical grounding, calibration, and DICOM integration with existing imaging software. In 2026, many systems support plug-and-play connectivity with AI-assisted alignment tools. Installation typically takes 4–6 hours, and clinics must ensure adequate space (minimum 2m x 2m), radiation shielding compliance, and network readiness prior to technician arrival. |
| 4. What warranty coverage is standard for panoramic X-ray units in 2026? | Most manufacturers provide a 2-year comprehensive warranty covering parts, labor, and X-ray tube performance. Extended warranties up to 5 years are available for purchase. In 2026, premium models include predictive maintenance alerts and remote diagnostics as part of warranty service. Always confirm whether the warranty is global or region-locked and whether on-site service response times are guaranteed (e.g., 48–72 hours). |
| 5. How are warranty claims handled, and what support is available during the coverage period? | Warranty claims are managed through the manufacturer’s authorized service network. Clinics should register their device online post-installation. Support includes 24/7 technical hotline, remote troubleshooting, and on-site repairs by certified engineers. In 2026, most brands offer mobile service apps for real-time tracking of repair status. Distributors are expected to assist with initial diagnostics and coordinate service dispatch. Keep all installation and service records for warranty validation. |
Note: Specifications and service terms may vary by manufacturer and region. Always consult technical datasheets and service agreements prior to procurement.
Need a Quote for Pano Machine Dental?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


