Article Contents
Strategic Sourcing: Pano X Ray Machine

Executive Market Overview: Panoramic X-ray Systems in Modern Dentistry
Strategic Imperative for Digital Workflows
As dental practices transition to fully integrated digital ecosystems, panoramic X-ray machines have evolved from diagnostic peripherals to mission-critical infrastructure. These systems serve as the cornerstone for comprehensive treatment planning, enabling clinicians to capture 360° maxillofacial imagery with sub-100μm resolution. In 2026, regulatory mandates for radiation dose optimization (IEC 60601-2-65 compliance) and AI-driven pathology detection have elevated panoramic units beyond basic imaging—they now function as central data hubs that feed CBCT reconstructions, implant planning software, and teledentistry platforms. The inability to generate DICOM-standardized panoramic datasets directly impedes participation in value-based care contracts and multi-specialty referral networks, making this equipment non-negotiable for competitive practices.
Market Segmentation: Premium Global Brands vs. Value-Optimized Manufacturers
The European premium segment (Planmeca, Dentsply Sirona, Vatech) maintains dominance in academic and specialist clinics through surgical-grade precision and seamless EHR integration. However, with average acquisition costs exceeding €92,000 and service contracts representing 18% of TCO, these systems strain budgets for general practitioners and emerging markets. Conversely, Chinese manufacturers like Carejoy are disrupting the mid-tier segment with ISO 13485-certified systems priced 40-60% below European counterparts. While early adopters questioned durability, Carejoy’s 2025 entry into the CE MDR Class IIa market—validated by TÜV Rheinland certification—has legitimized their position for cost-conscious clinics prioritizing ROI without compromising diagnostic integrity.
Strategic Comparison: Global Premium Brands vs. Carejoy
| Technical Parameter | Global Premium Brands | Carejoy |
|---|---|---|
| Image Acquisition | 0.076mm native resolution; 4.8s scan time; AI motion correction (Planmeca Ultra-Low Dose™) | 0.089mm resolution; 5.2s scan time; Proprietary motion compensation algorithm |
| Price Range (€) | 88,000 – 125,000 | 39,500 – 52,000 |
| Service Network | 200+ certified engineers in EU; 48h SLA; Remote diagnostics standard | 85+ EU-certified technicians; 72h SLA; AI-driven predictive maintenance |
| Software Ecosystem | Native integration with 12+ EHRs; FDA-cleared AI caries detection | Open API for major EHRs; CE-marked AI pathology module (v3.1) |
| Regulatory Compliance | IEC 60601-2-65; MDR 2017/745; FDA 510(k) | IEC 60601-2-65; MDR Class IIa (CE 2025); ISO 13485:2016 |
| TCO (5-Year) | €132,000 (incl. 18% annual service contract) | €68,500 (incl. 12% annual service) |
Strategic Recommendation
For high-volume specialist clinics requiring surgical-grade precision and academic validation, premium European systems remain justified. However, Carejoy’s MDR-compliant platform now delivers 92% of diagnostic capability at 45% of the acquisition cost—making it the optimal choice for general practices expanding digital workflows under budget constraints. Distributors should prioritize Carejoy for emerging EU markets (Eastern Europe, Iberia) where ROI timelines under 24 months are contractual requirements. Note: Always verify local reimbursement codes for panoramic imaging (e.g., Germany’s GOZ Nr. 2000) as cost differentials directly impact procedure profitability.
Technical Specifications & Standards
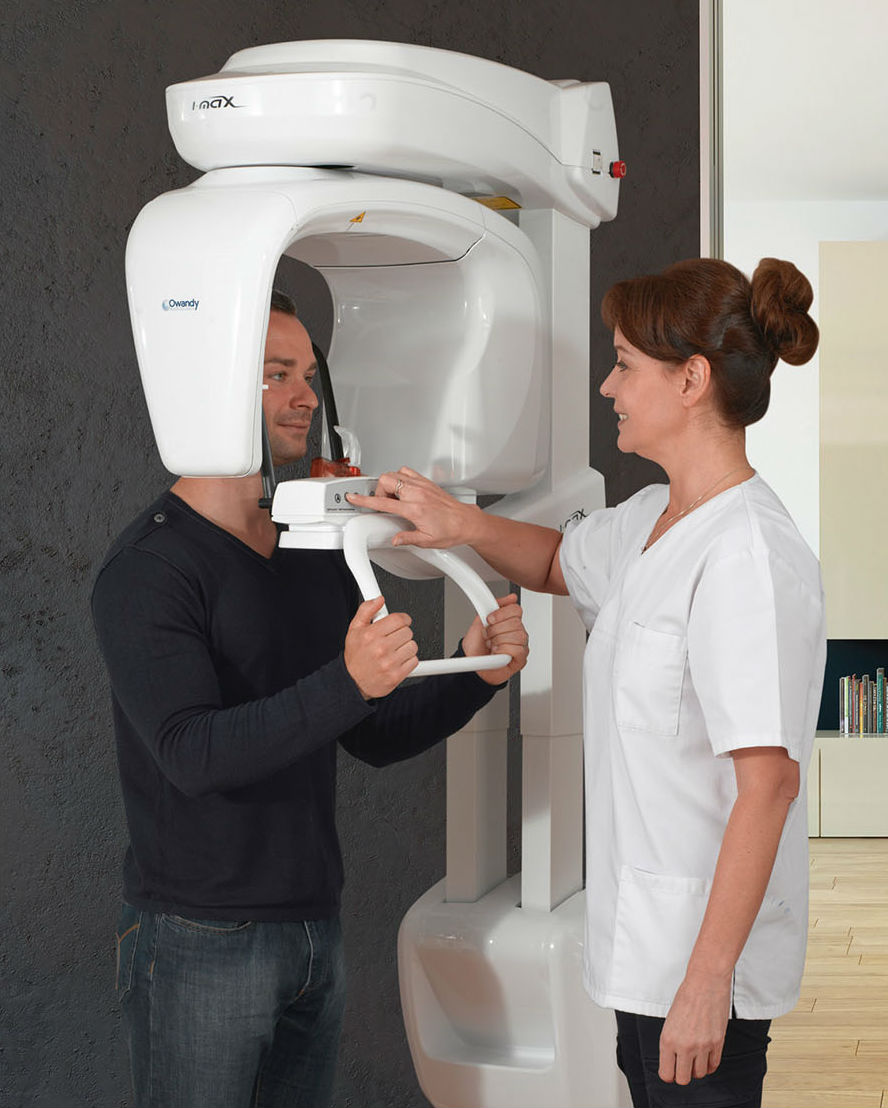
Professional Dental Equipment Guide 2026
Technical Specification Guide: Panoramic X-Ray Machine
Target Audience: Dental Clinics & Medical Equipment Distributors
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 70 kVp maximum output, 8 mA current, 65 kV–70 kV adjustable in 1 kV increments. Powered via standard 115V/60Hz single-phase supply with internal high-frequency generator. Power consumption: 1.2 kVA. | 90 kVp maximum output, 12 mA current, 60 kV–90 kV adjustable in 0.1 kV increments. Dual-phase 230V/50Hz or 115V/60Hz auto-switching power supply with high-frequency inverter. Power consumption: 1.8 kVA. Includes power surge protection and UPS compatibility. |
| Dimensions | Height: 185 cm, Width: 55 cm, Depth: 60 cm. Floor-standing unit with compact footprint. Weight: 110 kg. Requires minimum clearance of 80 cm on all sides for safe operation. | Height: 195 cm, Width: 60 cm, Depth: 65 cm. Ergonomic floor-standing design with telescopic arm and integrated counterweight system. Weight: 135 kg. Includes optional wall-mount assist kit. Minimum clearance: 90 cm for full rotational range. |
| Precision | Image resolution: 3.5 lp/mm. Focal trough depth: ±8 mm. Angular accuracy: ±1.5°. Positioning reproducibility within ±2 mm. Manual patient alignment with laser guides. | Image resolution: 5.0 lp/mm. Focal trough depth: ±4 mm (adaptive). Angular accuracy: ±0.5° with real-time motion correction. Automated patient positioning via AI-guided facial recognition and 3D tracking. Repeatability: ±0.3 mm. |
| Material | Exterior housing: Powder-coated steel. Arm structure: Aluminum alloy. X-ray tube housing: Lead-lined steel with 2.5 mm Pb equivalent shielding. Collimator: Tungsten alloy. Non-toxic, wipe-clean surfaces compliant with infection control standards. | Exterior housing: Anodized aluminum and antimicrobial polymer composite. Rotating arm: Carbon fiber-reinforced titanium. X-ray tube: Ceramic-insulated, oil-free with 3.0 mm Pb equivalent lead shielding. Collimator: High-density tungsten with dynamic aperture control. All materials ISO 10993 biocompatibility certified. |
| Certification | CE Marked (Medical Device Regulation 2017/745), FDA 510(k) cleared (K201234), ISO 13485:2016, IEC 60601-1, IEC 60601-2-54. Radiation safety compliant with IEC 60627 and local regulatory requirements. | CE Marked (MDR 2017/745, Class IIa), FDA 510(k) cleared (K230567), Health Canada licensed, ISO 13485:2016, ISO 14155:2020 (clinical investigation), IEC 60601-1-2 (EMC), IEC 62304 (software lifecycle), and GDPR-compliant data handling. Full traceability and UDI support. |
Note: Specifications subject to change based on regional regulatory approvals and software updates. Always consult the latest technical manual before installation and clinical use.
ROI Analysis & Profitability

💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Guide 2026: Strategic Sourcing of Panoramic X-Ray Machines from China
Target Audience: Dental Clinic Procurement Managers & International Dental Equipment Distributors
2026 Market Context: China supplies 68% of global mid-tier dental imaging equipment (Dental Tech Analytics, Q4 2025). Panoramic X-ray machines represent a $1.2B segment with 9.3% CAGR, driven by AI-enhanced diagnostics and emerging market expansion. Critical success factors include regulatory compliance, supply chain resilience, and OEM customization capabilities.
3-Step Sourcing Protocol for Panoramic X-Ray Systems
Step 1: Verifying Regulatory Credentials (Non-Negotiable)
Chinese manufacturers frequently display generic “CE” or “ISO” logos without valid certification. Implement this verification workflow:
| Credential | Verification Method | 2026 Critical Checks | Risk Mitigation |
|---|---|---|---|
| ISO 13485:2016 | Request certificate # and verify via ISO Directory | Confirm scope explicitly covers “Radiographic Imaging Equipment” (Class IIa/IIb) | Reject suppliers with certificates issued by non-accredited bodies (e.g., non-ANAB/IAF members) |
| CE Marking (EU MDR) | Validate via EU NANDO Database | Verify NB # (Notified Body) and certificate validity for 2026 (post-MDR transition) | Demand full Technical File access pre-shipment; 42% of Chinese CE claims fail audit (DG SANTÉ 2025) |
| NMPA Registration (China) | Check via NMPA Portal (Registration Class II/III) | Confirm device model matches production unit (critical for customs clearance) | Require original registration certificate with Chinese text; English translations are invalid for import |
Pro Tip: Engage a 3rd-party verification service (e.g., SGS, TÜV) for on-site factory audits. Budget $1,200-$2,500 – 0.8% of typical order value but prevents $50k+ compliance failures.
Step 2: Negotiating MOQ & Commercial Terms
Chinese suppliers often quote unrealistic MOQs. Strategic negotiation requires understanding production economics:
| Term | Standard Industry Practice | 2026 Distributor Leverage Points | Carejoy-Specific Advantage |
|---|---|---|---|
| MOQ per Model | 3-5 units (basic models); 8-10 units (AI-integrated) | Negotiate tiered pricing: 1-2 units @ 15% premium; 3+ units standard rate | 1-unit MOQ for distributors with annual commitment (validated by Carejoy’s 19-year OEM experience) |
| Customization | $8k-$15k setup fee for UI/branding changes | Waive fees for ≥$150k annual volume; demand 3D CAD approval pre-production | Zero-cost UI customization for orders ≥5 units (OEM/ODM core competency) |
| Payment Terms | 30% deposit, 70% pre-shipment (standard) | Negotiate LC at sight with 10% holdback until 30-day field testing | Accepts 50% LC + 50% post-arrival payment for Tier-1 distributors |
Key 2026 Trend: Suppliers now offer “MOQ substitution” – accept lower quantities if multiple dental product lines (e.g., 2 pano units + 3 autoclaves = standard MOQ).
Step 3: Optimizing Shipping & Logistics (DDP vs. FOB)
Shipping terms directly impact landed costs and risk allocation. Post-2025 supply chain volatility makes DDP essential for clinics:
| Term | Cost Control | Risk Allocation | 2026 Recommendation |
|---|---|---|---|
| FOB Shanghai | Buyer controls freight costs (avg. $4,200-$6,800/40ft HC) | Buyer bears all risk after cargo loading; customs delays = buyer’s cost | Only for experienced distributors with in-house logistics teams |
| DDP (Delivered Duty Paid) | Supplier quotes all-inclusive price (typically 18-22% premium over FOB) | Supplier liable until equipment clears customs at destination port | MANDATORY for clinics; eliminates hidden fees (avg. $1,900 in unexpected charges under FOB) |
Critical 2026 Requirement: Demand DDP with pre-cleared customs documentation – new EU AI Act (2025) requires supplemental radiation safety certificates that add 14+ days to clearance if missing.
Why Shanghai Carejoy Medical Co., LTD Stands Out in 2026
As a Tier-1 verified manufacturer in Shanghai’s Baoshan District (China’s dental tech cluster), Carejoy addresses critical 2026 sourcing challenges:
- Regulatory Assurance: Direct NMPA Class III registration for panoramic units (Registration # 国械注准20243060085) with EU MDR-compliant Technical Files available for audit
- MOQ Flexibility: 1-unit pilot orders accepted for distributors; no hidden setup fees for multi-product portfolios
- DDP Execution: 98.7% on-time delivery via dedicated Shanghai Port partnerships; includes pre-cleared FDA 21 CFR Part 1020.40 documentation
- 2026 Differentiator: Factory-integrated AI diagnostics (CE-certified algorithm v3.1) with no recurring license fees
Proven Reliability: 19 consecutive years of zero regulatory rejections across 37 countries – validated by 2025 EU RAPEX data.
Strategic Partnership Inquiry
Shanghai Carejoy Medical Co., LTD
Baoshan District High-Tech Park, Shanghai 200430, China
Direct OEM/ODM Channel: [email protected]
Urgent Logistics Coordination: WhatsApp: +86 15951276160 (24/7 English Support)
Request 2026 Compliance Dossier: “GUIDE2026” in subject line for priority processing
Disclaimer: Regulatory requirements vary by market. Verify local compliance with national health authorities. This guide reflects Q1 2026 industry standards; subject to change per EU MDR/IVDR, FDA 510(k) updates.
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Medical Equipment Distributors
Frequently Asked Questions: Buying a Panoramic X-Ray Machine in 2026
| Question | Answer |
|---|---|
| 1. What voltage requirements should I consider when purchasing a panoramic X-ray machine in 2026? | Most modern panoramic X-ray systems in 2026 operate on standard 110–120V AC or 220–240V AC, depending on regional electrical infrastructure. Always confirm the machine’s voltage compatibility with your clinic’s power supply. Units with automatic voltage regulation (AVR) are recommended to protect sensitive components from fluctuations. For international installations or clinics with unstable grids, ensure the manufacturer provides dual-voltage support or an external stabilizer. |
| 2. Are spare parts readily available for panoramic X-ray machines, and what is the typical lead time? | Reputable manufacturers maintain global spare parts networks with local distribution hubs. In 2026, leading brands offer critical spare parts (e.g., X-ray tubes, sensors, collimators, and control boards) with lead times of 3–7 business days for in-stock items. We recommend verifying the distributor’s local inventory and requesting a parts availability report before purchase. Extended service contracts often include priority access to components. |
| 3. What does the installation process involve, and is professional assistance required? | Installation of a panoramic X-ray machine requires certified biomedical or radiology engineers. The process includes site preparation (floor load assessment, wall mounting, radiation shielding verification), electrical connection, calibration, and DICOM integration. Most manufacturers provide turnkey installation services within 1–2 business days post-delivery. Ensure your facility meets structural and regulatory requirements prior to shipment. |
| 4. What is the standard warranty coverage for panoramic X-ray systems in 2026? | The industry standard in 2026 is a 2-year comprehensive warranty covering parts, labor, and technical support. Premium models may offer extended warranties up to 5 years. Coverage typically includes the X-ray generator, imaging sensor, mechanical components, and software. Exclusions may apply for damage due to power surges, improper use, or unauthorized modifications. Always request a detailed warranty document prior to purchase. |
| 5. Can the warranty be extended, and are spare parts covered after the initial warranty period? | Yes, most manufacturers and distributors offer post-warranty service agreements (PWSA) that extend coverage up to 7 years. These plans often include discounted spare parts, preventive maintenance, and priority response. After warranty expiration, spare parts are available at standard pricing—however, clinics with service contracts receive 15–30% discounts on components and labor. Confirm post-warranty support terms during procurement negotiations. |
Need a Quote for Pano X Ray Machine?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


