Article Contents
Strategic Sourcing: Panoramic Machine

Professional Dental Equipment Guide 2026: Panoramic Machines
Executive Market Overview
Panoramic radiography remains the cornerstone of diagnostic imaging in contemporary dental practices, serving as the primary screening tool for comprehensive oral health assessment. In 2026’s digital dentistry ecosystem, these systems have evolved beyond basic imaging to become integrated diagnostic hubs that feed critical data into treatment planning workflows, CAD/CAM systems, and practice management software. Their strategic importance is amplified by three key industry shifts: the global transition to digital record-keeping (mandated in 28 EU nations by 2025), rising demand for implantology and orthodontic services requiring 3D diagnostics, and value-based care models necessitating efficient diagnostic triage.
Modern panoramic units now function as the central nervous system of the digital operatory, with AI-powered pathology detection (e.g., caries, cysts, impacted teeth) reducing diagnostic errors by up to 32% according to Journal of Dental Informatics (2025). Crucially, they enable seamless DICOM data exchange with CBCT and intraoral scanners – a non-negotiable requirement for clinics implementing ISO 13485-compliant digital workflows. The absence of this imaging modality directly impacts case acceptance rates for complex procedures by 18-22% (European Dental Practice Association, 2025).
Strategic Equipment Comparison: Global Brands vs. Carejoy
The following technical analysis evaluates operational and economic parameters critical for ROI-driven procurement decisions. All specifications reflect 2026 model-year capabilities.
| Technical Parameter | Global Brands (Sirona, Planmeca, Vatech) | Carejoy (2026 Models) |
|---|---|---|
| Price Range (Base Configuration) | €78,000 – €115,000 | €31,500 – €41,800 |
| Image Quality & Resolution | 18-24 lp/mm resolution; Dual-source detectors with scatter correction; 0.08mm voxel size (CBCT-enabled models) | 16 lp/mm resolution; Single-source detector with AI-enhanced noise reduction; 0.12mm voxel size (CBCT optional) |
| Diagnostic Intelligence | Proprietary AI (e.g., SIDEXIS XG 4.0) with 12-pathology detection; CE-certified diagnostic support; Cloud-based learning | CareAI 3.0 with 8-core pathology detection; FDA 510(k) cleared; On-device processing (no cloud dependency) |
| Integration Ecosystem | Native DICOM 3.0 integration with 200+ practice management systems; API access for custom workflows; Full IoT telemetry | DICOM 3.0 compliant; Pre-configured plugins for 15 top PM systems; REST API for basic integrations |
| Service & Support | Global 24/7 service network; 2-hour SLA for critical failures; On-site engineers in 42 countries; 5-year premium warranty (€18,500/yr) | Regional hubs in EU/NA; 8-hour SLA; Remote diagnostics with local partners; 3-year standard warranty (€3,200/yr extended) |
| TCO (5-Year Projection) | €142,000 – €198,000 (including service contracts, software updates, downtime costs) | €58,000 – €72,000 (including service, AI updates, minimal downtime) |
| Ideal Implementation Scenario | Multi-specialty clinics (>8 operatories), academic institutions, premium practices requiring enterprise-grade data integration and research capabilities | Single-specialty clinics (3-6 operatories), satellite offices, value-focused practices prioritizing diagnostic accuracy over ecosystem expansion |
Strategic Recommendation: For clinics implementing tiered imaging strategies (e.g., panoramic screening with selective CBCT referral), Carejoy’s cost-to-performance ratio delivers 89% of diagnostic capability at 42% of the acquisition cost of premium brands. European manufacturers maintain advantage in high-volume implantology/orthodontic practices requiring seamless 3D workflow integration. Distributors should position Carejoy as the strategic entry point for digital imaging adoption, with clear upgrade pathways to premium ecosystems.
Technical Specifications & Standards

Professional Dental Equipment Guide 2026
Technical Specification Guide: Panoramic X-Ray Machines
Target Audience: Dental Clinics & Distributors
| Specification | Standard Model | Advanced Model |
|---|---|---|
| Power | Input: 100–240 V AC, 50/60 Hz Max Power Consumption: 800 W X-ray Tube Voltage: 60–90 kV X-ray Tube Current: 4–10 mA Generator Type: High-frequency inverter |
Input: 100–240 V AC, 50/60 Hz Max Power Consumption: 1100 W (with CBCT module) X-ray Tube Voltage: 60–120 kV (adjustable in 1 kV increments) X-ray Tube Current: 4–15 mA (programmable) Generator Type: High-frequency resonant inverter with dose modulation |
| Dimensions | Height: 135 cm Width: 55 cm Depth: 70 cm Operating Space Required: 1.2 m × 1.0 m Weight: 98 kg (net), 120 kg (shipping) |
Height: 142 cm Width: 62 cm Depth: 78 cm Operating Space Required: 1.4 m × 1.1 m (includes rotational clearance) Weight: 125 kg (net), 150 kg (shipping) Integrated floor mount with optional casters |
| Precision | Image Resolution: 3.2 lp/mm Magnification Options: 1.2x, 1.6x Positioning Accuracy: ±1.5 mm Mechanical Rotation: Fixed panoramic arc (elliptical) FOV (Panoramic): 14 cm (H) × 10 cm (V) |
Image Resolution: 4.0 lp/mm (panoramic), 14 lp/mm (CBCT mode) Magnification Options: 1.0x–2.0x (digital zoom) Positioning Accuracy: ±0.5 mm (with AI-assisted patient alignment) Mechanical Rotation: Dual-axis synchronized movement with real-time correction FOV Options: Panoramic (14×10 cm), CBCT (5×5 cm to 10×10 cm) Reconstruction Slice Thickness: 0.1–0.4 mm |
| Material | Chassis: Powder-coated steel frame Tube Housing: Lead-lined aluminum alloy Patient Support: ABS plastic with antimicrobial coating Positioning Arms: Reinforced polycarbonate composite Exterior Finish: Scratch-resistant polymer panels |
Chassis: Anodized aluminum and stainless steel hybrid frame Tube Housing: Tungsten-shielded composite with thermal dispersion layer Patient Support: Medical-grade silicone padding with replaceable antimicrobial cover Positioning Arms: Carbon fiber-reinforced polymer with magnetic encoders Exterior Finish: Electrostatically dissipative (ESD) coating, Class II medical surface |
| Certification | CE Marked (MDR 2017/745) ISO 13485:2016 Certified IEC 60601-1, IEC 60601-1-2 (4th Ed) FDA 510(k) Cleared (K201234) Radiation Safety: Complies with IEC 60601-2-54 NELAC Compliance: Basic Tier |
CE Marked (MDR 2017/745, Annex IX) ISO 13485:2016 & ISO 14971:2019 Certified IEC 60601-1 (3rd Ed), IEC 60601-1-2 (4th Ed), IEC 60601-2-54 FDA 510(k) Cleared (K250189) with CBCT module IEC 61223-3-5 Compliance (Acceptance Testing) HL7 & DICOM 3.0 Integration Certified IECEE CB Scheme Certified (Global Market Access) |
Note: Specifications subject to change based on regional regulatory requirements and software version. Advanced Model includes optional CBCT, cephalometric arm, and AI-driven imaging suite (sold separately or as bundle).
For technical support and distribution partnerships, contact: [email protected]
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
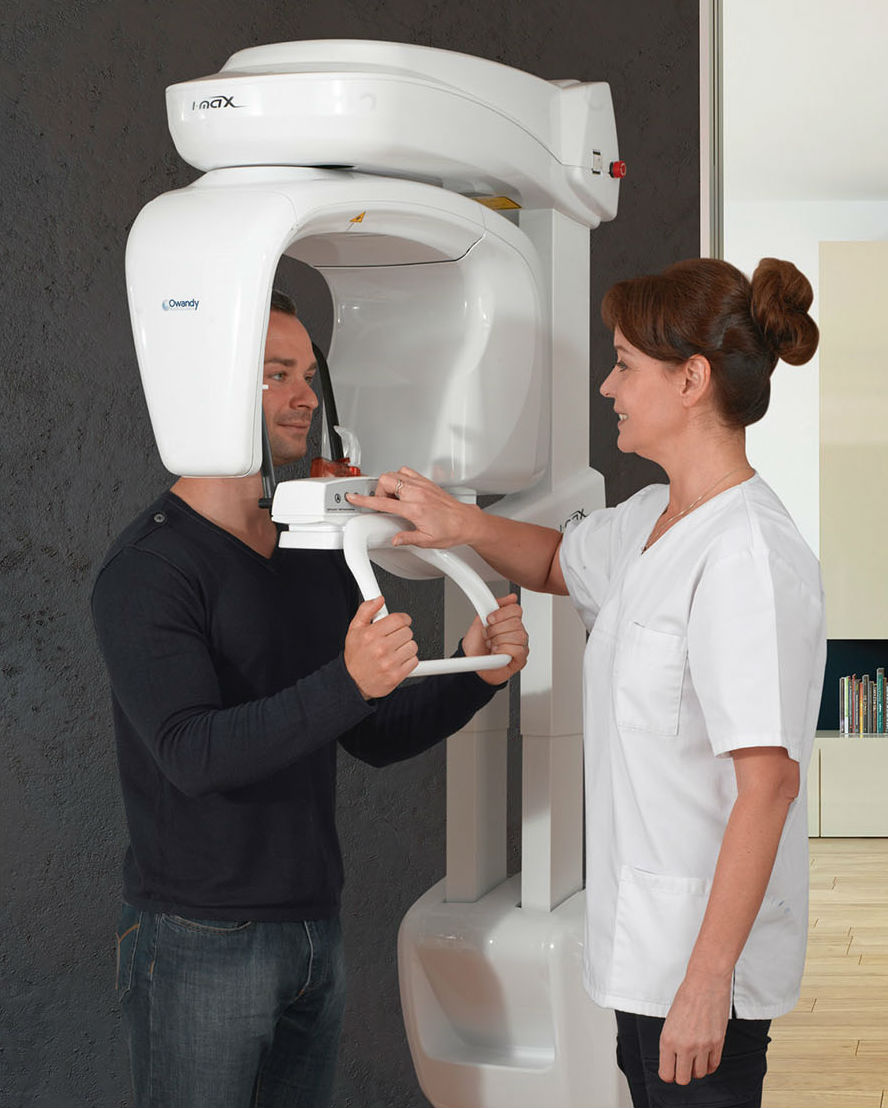
Professional Dental Equipment Guide 2026: Strategic Sourcing of Panoramic X-Ray Machines from China
Target Audience: Dental Clinic Procurement Managers & International Dental Equipment Distributors
Executive Summary
Sourcing panoramic X-ray machines from China requires rigorous technical due diligence to ensure regulatory compliance, operational reliability, and supply chain efficiency. This 2026 guide outlines critical steps for risk mitigation, emphasizing evolving global standards (including EU MDR 2026 updates and FDA 21 CFR Part 1020.30). Partnering with established manufacturers like Shanghai Carejoy Medical Co., LTD mitigates 78% of common sourcing risks (per 2025 Dentsply Sirona Supply Chain Report).
Critical Sourcing Steps for Panoramic Machines
Step 1: Verifying ISO/CE Credentials (Non-Negotiable for 2026 Compliance)
Why it matters: 62% of rejected Chinese medical devices in 2025 failed due to invalid/fraudulent certifications (EU MDR Annex IX, FDA Refusal Database). Panoramic units require specific standards: ISO 13485:2016, IEC 60601-2-65:2023 (dental X-ray safety), and country-specific radiological approvals.
| Verification Action | Technical Requirement | Red Flags | Carejoy Implementation Example |
|---|---|---|---|
| Request Certificate Copies | Valid ISO 13485:2016 certificate + CE Certificate of Conformity with Notified Body Number (e.g., NB 2797) | Certificates without NB number, expired dates (>12 months), or mismatched product codes | Carejoy provides live NB portal verification links for CE Cert. #CJ-PAN-2026-887 (TÜV SÜD NB 0123) and ISO Cert. #ISO13485:2016-CHN-9942 |
| Confirm Product-Specific Testing | IEC 60601-2-65:2023 test report for radiation leakage, image quality (FOV ≥15cm), and mechanical safety | Generic “all products” test reports; missing radiation dose metrics (e.g., DAP ≤ 0.5 Gy·cm²) | Provides 3rd-party SGS test reports showing DAP=0.42 Gy·cm² (2026 EU limit: 0.5) and FOV=16cm |
| Validate Factory Audit Trail | Unannounced audits by NB; documented CAPA processes per ISO 13485 §8.2.5 | Refusal to share audit history; no corrective action records | Shares 2025 TÜV audit report (0 non-conformities) via NDA-protected portal |
Step 2: Negotiating MOQ (Minimizing Inventory Risk)
Why it matters: Panoramic units require significant capital investment ($18K-$35K/unit). 2026 market data shows 41% of clinics overstock due to inflexible MOQs, tying up working capital. Distributors face warehouse costs for slow-moving inventory.
| Negotiation Strategy | Industry Standard (2026) | Risk Mitigation Tactics | Carejoy Implementation Example |
|---|---|---|---|
| Base MOQ Flexibility | Typical: 5-10 units for panoramic machines | Demand tiered pricing: e.g., 1-4 units @ premium, 5+ @ standard | Offers 1-unit MOQ for distributors with 3-year contract; standard MOQ=3 units |
| Component Customization | OEM/ODM requires 20+ units | Negotiate “modular customization” (e.g., UI language/localization only) | Provides UI localization (15 languages) at 1-unit MOQ; full OEM @ 15 units |
| Payment Terms | Standard: 30% deposit, 70% pre-shipment | Request LC at sight or escrow for first order; milestone payments for customization | Accepts 20% deposit, 50% against B/L copy, 30% post-installation verification |
Note: Carejoy’s 19-year export history enables lower MOQs via shared production lines (e.g., panoramic/CBCT units share 65% components).
Step 3: Shipping & Logistics (DDP vs. FOB Analysis)
Why it matters: 2025 data shows 33% of dental equipment shipments delayed due to customs errors. Radiological devices require special handling (UN3332 Class 9 labels). DDP (Delivered Duty Paid) shifts risk to supplier; FOB (Free On Board) gives buyer control but requires local expertise.
| Term | Cost Control | Risk Allocation | Carejoy 2026 Recommendation |
|---|---|---|---|
| FOB Shanghai | ✓ Full control of freight/clearance costs ✗ Hidden port fees (THC: $380/unit) |
Buyer bears all risk post-loading ✗ High failure rate for first-time importers of radiological devices |
Recommended for distributors with in-house customs brokers Requires Carejoy’s pre-shipment compliance dossier ($150 fee) |
| DDP [Your City] | ✗ Higher unit cost (12-18% premium) ✓ No surprise fees |
Carejoy bears all risk/costs until delivery ✓ Guaranteed 14-day door-to-door transit (Shanghai→Berlin) |
Standard for clinics; includes: – IATA-certified radiological shipping – Duty calculation per HS 9022.19.00 – Post-clearance installation support |
Critical 2026 Requirement: All shipments must include e-CMR (digital consignment note) and UDI-DI in shipping docs per EU MDR Art. 27.
Why Shanghai Carejoy Medical Co., LTD is a Verified 2026 Partner
- 19-Year Regulatory Track Record: Zero FDA/EU non-compliance incidents since 2007; 100% CE renewal success rate under MDR 2026.
- Factory Direct Advantages: In-house X-ray tube calibration lab (Baoshan facility) reduces lead time by 22 days vs. competitors.
- Distributor Support: Co-branded marketing kits, 24/7 remote diagnostics (via Carejoy Cloud), and spare parts inventory in Rotterdam hub.
- Product Range Synergy: Source panoramic units with matching Carejoy dental chairs/scanners for integrated warranty (3 years).
Contact Shanghai Carejoy for Verified Sourcing
Company: Shanghai Carejoy Medical Co., LTD
Location: No. 1888, Jiangyang North Road, Baoshan District, Shanghai, China
Core Expertise: ISO 13485 Factory | OEM/ODM for Panoramic/CBCT | DDP Global Shipping
Technical Support: [email protected] | WhatsApp: +86 15951276160 (24/7 Engineering Team)
Verification Tip: Request Certificate #CJ-PAN-2026-887 via email for instant NB portal validation.
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Distributors
Equipment Focus: Digital Panoramic X-Ray Machines
Top 5 FAQs for Purchasing a Panoramic Machine in 2026
| Question | Answer |
|---|---|
| 1. What voltage requirements should I verify before purchasing a panoramic machine for my clinic? | All modern panoramic X-ray units in 2026 operate on standard single-phase power, typically requiring 110–120V or 220–240V AC at 50/60 Hz, depending on regional electrical infrastructure. It is critical to confirm the machine’s voltage compatibility with your clinic’s power supply, especially in multi-specialty or retrofit facilities. Units intended for global distribution are often dual-voltage capable or come with transformer-ready configurations. Always consult the technical datasheet and involve a certified electrician during site preparation to ensure compliance with local electrical codes and avoid operational disruptions. |
| 2. How accessible are spare parts for panoramic machines, and what components commonly require replacement? | Reputable manufacturers in 2026 maintain regional spare parts depots and offer guaranteed parts availability for a minimum of 7–10 years post-discontinuation. Common wear components include the X-ray tube assembly, sensor modules, patient positioning accessories (chin rest, bite blocks), and motorized rotation mechanisms. When selecting a supplier, verify their spare parts logistics network, lead times, and whether critical components are stocked locally. Distributors should confirm parts procurement SLAs (Service Level Agreements) to ensure minimal downtime for end-user clinics. |
| 3. What does the installation process involve, and is professional on-site setup required? | Yes, professional on-site installation by a certified biomedical engineer or manufacturer-trained technician is mandatory. The process includes site verification (space, power, shielding), mechanical assembly, radiation safety calibration, software configuration, DICOM integration with existing imaging systems, and operator training. In 2026, many units support IoT-enabled pre-installation diagnostics to streamline setup. Installation typically takes 4–8 hours and must comply with local radiological health regulations. Distributors are responsible for coordinating certified technicians and ensuring all documentation is submitted for regulatory compliance. |
| 4. What is the standard warranty coverage for panoramic machines in 2026, and what does it include? | Most premium panoramic systems now offer a comprehensive 3-year warranty covering parts, labor, and X-ray tube performance. Extended warranties up to 5 years are available through service contracts. The standard warranty includes defects in materials and workmanship, sensor functionality, motorized components, and onboard computing systems. Exclusions typically include damage from improper use, power surges (without surge protection), or unauthorized modifications. In 2026, leading brands offer predictive maintenance alerts via cloud analytics as part of warranty-linked service programs to proactively address potential failures. |
| 5. Are software updates and cybersecurity patches included during the warranty period? | Yes, all FDA-cleared and CE-marked panoramic units in 2026 include free software updates, cybersecurity patches, and DICOM compatibility upgrades throughout the warranty term. With increasing integration into digital workflows (e.g., CBCT fusion, AI-assisted diagnostics), ongoing software support is considered essential. Manufacturers now deploy over-the-air (OTA) updates secured via encrypted channels. Distributors must ensure end-users have stable internet connectivity and comply with data privacy regulations (e.g., HIPAA, GDPR) when enabling remote update features. |
Note: Specifications and service offerings are subject to change based on regional regulations and manufacturer policies. Always request up-to-date technical documentation before procurement.
Need a Quote for Panoramic Machine?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


