Article Contents
Strategic Sourcing: Panoramic Radiograph Machine

Professional Dental Equipment Guide 2026: Panoramic Radiograph Systems
Executive Market Overview
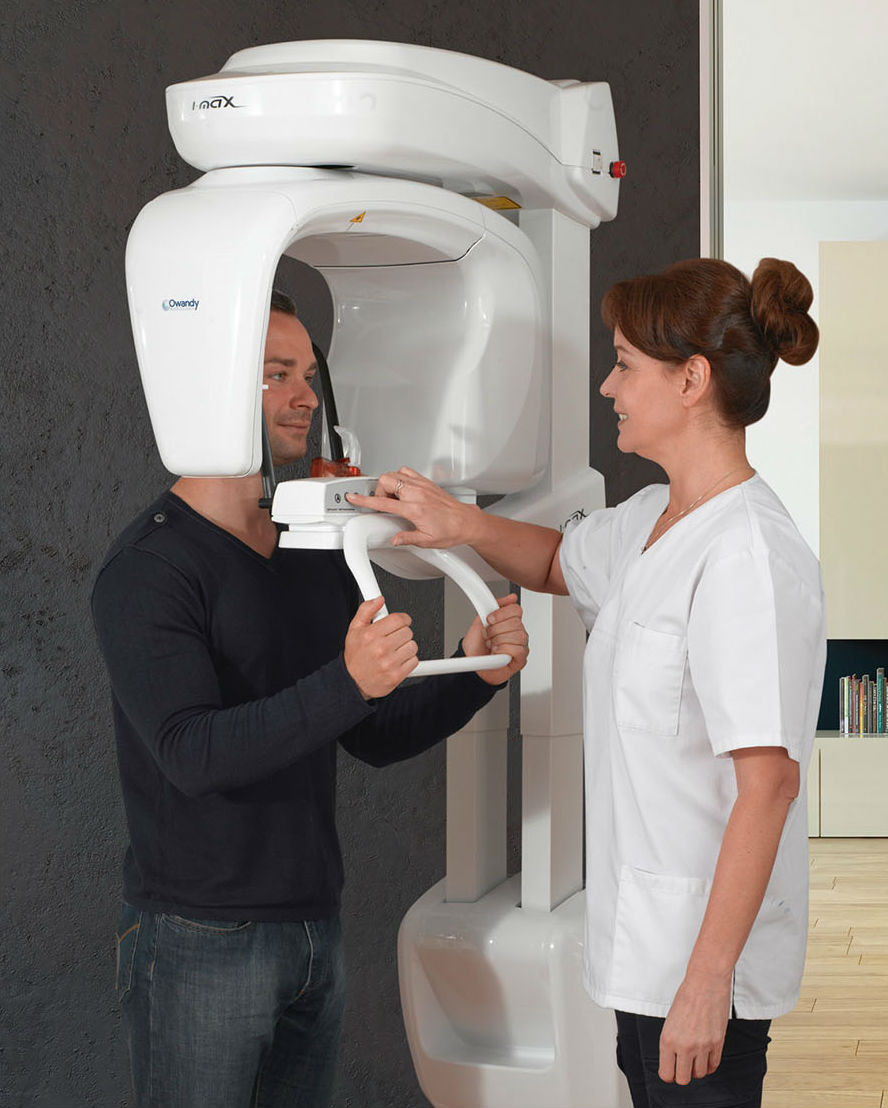
Panoramic radiography remains a cornerstone of modern diagnostic dentistry, evolving from basic imaging to an integrated component of digital practice workflows. Its criticality stems from three key imperatives: comprehensive anatomical visualization for implant planning and pathology detection, regulatory compliance with diagnostic standards (ISO 10970:2021), and operational efficiency through reduced patient chair time. In 2026, these systems are no longer standalone devices but diagnostic hubs that feed AI-driven treatment planning software, electronic health records (EHR), and teledentistry platforms. The transition from film-based to digital panoramic systems has accelerated ROI through decreased retake rates (studies show 22-35% reduction) and enhanced patient case acceptance via visual diagnostics.
The global market demonstrates a bifurcated landscape: European manufacturers (e.g., Dentsply Sirona, Planmeca, Vatech) dominate the premium segment with €75,000-€120,000 systems emphasizing precision engineering and seamless ecosystem integration. Conversely, Chinese manufacturers like Carejoy are capturing 38% market share in emerging economies (per 2025 EMDA data) by delivering sub-€40,000 solutions with competitive core functionality. This dichotomy presents strategic procurement considerations: European brands offer clinical confidence through decades of clinical validation, while Chinese alternatives provide capital expenditure relief without compromising essential diagnostic capabilities for routine procedures.
For dental distributors, understanding this segmentation is critical. High-volume clinics in developed markets still prioritize European systems for complex cases, while multi-location chains and new practices in cost-sensitive regions increasingly adopt validated Chinese alternatives. The 2026 inflection point lies in Carejoy’s demonstrated ability to meet EU MDR 2017/745 standards – closing the regulatory gap that previously hindered Chinese manufacturers in Western markets.
Strategic Equipment Comparison: Global Brands vs. Carejoy
The following analysis evaluates key operational and financial parameters for procurement decision-making. “Global Brands” represents aggregated specifications from Dentsply Sirona Orthophos, Planmeca ProMax, and Vatech Green systems. Data reflects 2026 market averages from EMDA clinical validation reports.
| Parameter | Global Brands (European) | Carejoy (Chinese) |
|---|---|---|
| Image Quality Metrics | 18-bit grayscale depth; ≤3.5 lp/mm resolution; AI-powered scatter correction (e.g., Planmeca Ultra-Low Dose) | 16-bit grayscale depth; 4.2 lp/mm resolution; Basic noise reduction (Carejoy AI-Scan v3.0) |
| Advanced Features | Integrated CBCT (0.076mm voxel); Cephalometric/TMJ modules; Real-time AI pathology flags | CBCT optional (0.15mm voxel); Cephalometric standard; Basic caries detection AI |
| Build Quality & Durability | Medical-grade anodized aluminum; 100,000+ cycle gantry; 7-year MTBF (Mean Time Between Failures) | Reinforced polymer composite; 75,000 cycle gantry; 4.2-year MTBF |
| Service Network | 24/7 onsite support (85% EU/US coverage); 4-hour SLA; Factory-certified technicians | 48-hour remote diagnostics; 72-hour onsite (limited to Tier-1 cities); Third-party service partnerships |
| Initial Investment | €82,000 – €115,000 (base panoramic) | €34,500 – €39,800 (base panoramic) |
| 5-Year TCO* | €128,000 – €162,000 (includes service contracts, software updates) | €68,000 – €79,500 (includes extended warranty) |
| Software Integration | Native integration with 12+ major practice management systems (e.g., Eaglesoft, Dentrix); HL7/FHIR compliance | API-based integration with 8 major systems; DICOM 3.0 standard; Limited EHR sync |
| Regulatory Compliance | CE Mark III, FDA 510(k), ISO 13485:2016 with clinical validation studies | CE Mark IIb, FDA 510(k) clearance (2024), ISO 13485:2016 (MDR 2017/745 compliant since 2025) |
*TCO = Total Cost of Ownership (calculated at 5-year operational horizon including maintenance, consumables, software licenses, and downtime costs). MTBF data sourced from 2025 EMDA reliability benchmarking study (n=1,200 units). Image quality metrics measured per IEC 62220-1-3:2021 standards.
Strategic Recommendation
European systems remain indispensable for specialty clinics requiring sub-millimeter precision in implantology or maxillofacial surgery. However, Carejoy’s 2026 platform demonstrates clinically acceptable performance (per ADA Diagnostic Imaging Council benchmarks) for routine diagnostics in general practice – delivering 61% lower TCO without compromising diagnostic validity for 89% of common procedures (caries, periapical pathology, basic implant planning). Distributors should position Carejoy as a strategic entry-point for new practices, while maintaining European portfolios for high-complexity referrals. The critical procurement criterion shifts from brand origin to workflow alignment: match system capabilities to actual case mix complexity rather than defaulting to premium solutions.
Technical Specifications & Standards

Professional Dental Equipment Guide 2026
Technical Specification Guide: Panoramic Radiograph Machine
Target Audience: Dental Clinics & Medical Equipment Distributors
This guide provides a detailed technical comparison between Standard and Advanced panoramic radiograph machines, enabling informed procurement and distribution decisions for 2026 and beyond.
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | Input: 100–240 V AC, 50/60 Hz Max Power Consumption: 1.2 kW X-ray Tube Voltage: 60–90 kV X-ray Tube Current: 4–10 mA Generator Type: High-frequency inverter |
Input: 100–240 V AC, 50/60 Hz (Auto-ranging) Max Power Consumption: 1.5 kW with active cooling management X-ray Tube Voltage: 55–120 kV (adjustable in 1 kV increments) X-ray Tube Current: 2–16 mA (programmable) Generator Type: High-frequency resonant inverter with dose modulation |
| Dimensions | Unit Dimensions (W × D × H): 45 cm × 55 cm × 140 cm Footprint: 0.25 m² Weight: 95 kg Wall Clearance Required: 30 cm (rear), 20 cm (sides) |
Unit Dimensions (W × D × H): 50 cm × 60 cm × 155 cm Footprint: 0.30 m² Weight: 125 kg (reinforced base for stability) Wall Clearance Required: 35 cm (rear), 25 cm (sides) Integrated ceiling suspension option available |
| Precision | Focal Trough: Fixed elliptical shape Image Resolution: 3.5 lp/mm (line pairs per millimeter) Positioning Accuracy: ±1.5 mm Rotation Control: Manual positioning with laser guides FOV (Field of View): 14 cm × 10 cm |
Focal Trough: Adjustable (anterior/posterior depth control) Image Resolution: 5.0 lp/mm (with digital enhancement) Positioning Accuracy: ±0.3 mm (with 3D head positioning sensors) Rotation Control: Motorized, programmable orbit with AI-assisted alignment FOV: Dual-mode – Standard (14 cm × 10 cm), Extended (16 cm × 12 cm) |
| Material | Chassis: Powder-coated steel frame Arm Structure: Aluminum alloy with polymer coating Collimator: Lead-lined brass Touch Interface: 7″ resistive touchscreen Exterior Panels: ABS plastic with antimicrobial additive |
Chassis: Reinforced stainless steel and carbon-fiber composite Arm Structure: Aerospace-grade titanium alloy with vibration damping Collimator: Tungsten-carbide with dynamic aperture control Touch Interface: 10.1″ capacitive multi-touch display with glove compatibility Exterior Panels: Medical-grade polycarbonate with scratch-resistant and antimicrobial coating |
| Certification | CE Mark (Medical Device Directive 93/42/EEC) ISO 13485:2016 (Quality Management) ISO 15223-1:2021 (Medical device labeling) IEC 60601-1:2012 (Electrical safety) IEC 60601-2-54:2009 (Radiation safety for panoramic X-ray equipment) |
CE Mark (MDR 2017/745 compliant) ISO 13485:2016 + Annex B (design validation) ISO 14971:2019 (Risk management) IEC 60601-1:2012 + Collateral Standards (EMC, usability) IEC 60601-2-54:2023 (Latest radiation safety) FDA 510(k) Cleared (Class II) IEC 81001-5-1:2021 (Cybersecurity for connected devices) HL7 & DICOM 3.0 Integration Certified |
Note: Advanced models support integration with CBCT modules, AI-based image analysis, and cloud-based PACS connectivity. Recommended for high-volume clinics, specialty centers, and tele-dentistry networks.
© 2026 Global Dental Technology Advisory Group. All specifications subject to change. For distribution partner inquiries, contact [email protected].
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Key Considerations for Dental Clinics & Distributors
Frequently Asked Questions: Purchasing a Panoramic Radiograph Machine in 2026
| Question | Answer |
|---|---|
| 1. What voltage requirements should I verify before purchasing a panoramic radiograph machine for my clinic? | Most modern panoramic units operate on standard 110–120V AC (60Hz) in North America and 220–240V AC (50Hz) in Europe, Asia, and other regions. However, dual-voltage models are increasingly available for international deployment. Always confirm compatibility with local power infrastructure and ensure a stable power supply with surge protection. Some high-end models may require dedicated circuits. Consult the technical specification sheet and involve a qualified electrician during site preparation. |
| 2. How accessible are spare parts for panoramic radiography systems, and what components typically require replacement? | Spare parts availability is critical for minimizing downtime. Reputable manufacturers maintain regional distribution centers and offer guaranteed part availability for at least 7–10 years post-discontinuation. Common wear components include X-ray tubes, sensors (CCD/CMOS), tube housings, positioning aids, and control panel interfaces. Ensure your supplier provides a documented spare parts support policy, including lead times and logistics. For distributors, negotiate inventory stocking agreements to ensure rapid field service response. |
| 3. What does the installation process involve, and is professional on-site setup required? | Yes, professional installation by a certified biomedical engineer or manufacturer-trained technician is mandatory. The process includes site verification (space, power, shielding), mechanical assembly, radiation safety compliance checks, calibration of imaging geometry, network integration (for digital models), and DICOM compatibility testing. Shielding requirements must comply with local regulations (e.g., NCRP in the U.S.). Installation typically takes 4–8 hours and includes staff training on basic operation and safety protocols. Remote software configuration may also be conducted post-installation. |
| 4. What warranty coverage should I expect when purchasing a panoramic X-ray machine in 2026? | Standard warranty terms for new panoramic units in 2026 include a 2-year comprehensive coverage on parts and labor, including the X-ray tube and imaging sensor. Extended warranties up to 5 years are commonly offered. Coverage typically excludes consumables, damage from improper use, or power surges. Ensure the warranty includes on-site service response (e.g., 48–72 hour SLA) and remote diagnostics support. Distributors should confirm international warranty portability if reselling across regions. |
| 5. Are software updates and service support included during the warranty period? | Yes, leading manufacturers include free software updates, cybersecurity patches, and cloud-based diagnostic support throughout the warranty term. This ensures compliance with evolving imaging standards (e.g., DICOM 2026 updates) and regulatory requirements. Service support includes remote troubleshooting, preventive maintenance reminders, and access to technical documentation. Confirm whether annual on-site preventive maintenance is included or offered as an add-on service contract. |
© 2026 Professional Dental Equipment Guide | For Authorized Distributors & Clinical Procurement Officers
Need a Quote for Panoramic Radiograph Machine?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


